MEETING HIGHLIGHT
Exploring TYK2-targeted approaches: Deucravacitinib demonstrates notable efficacy and tolerability as a first-in-class treatment for psoriasis
Psoriasis (PsO) incurs tremendous disease burden due to persistent symptoms and ineffective treatment strategies.1 Advancements in the understanding of PsO pathogenesis have led to the identification of tyrosine kinase 2 (TYK2) as a key player in the signaling of interleukin 23 (IL-23) and related cytokines.2,3 Building upon this knowledge, the development of TYK2-targeted therapies has emerged, with deucravacitinib as the first-in-class TYK2 inhibitor approved for the treatment of PsO.4 During a recent symposium, Dr. Linda Stein Gold described the role of TYK2 in the treatment of PsO, illustrated the clinical efficacy and safety profile of deucravacitinib in patients with moderate-to-severe PsO and highlighted its simple dosing regimen and initiation requirements, cementing its role as an optimal treatment option for PsO.
Unresolved treatment burden among PsO patients
PsO is an immune-mediated inflammatory skin disease characterized by persistent inflammation, leading to abnormal proliferation and differentiation of keratinocytes.5 The Global Burden of Disease (GBD) 2019 study reported a global PsO incidence of roughly 4.6 million in 2019, highlighting the significant global burden of this disease.6
Nevertheless, treatment outcomes for PsO remain suboptimal, with patients under-treated or dissatisfied with their current therapies.1 Findings from the UPLIFT survey revealed that 42% of patients with moderate PsO (body surface area [BSA] 4-10 palms) did not receive any systemic treatment, with 16% remaining untreated.1 Among patients with severe PsO (BSA>10 palms), 43% did not receive any systemic treatment, while 12% remained untreated.1
Furthermore, a significant proportion of patients in the UPLIFT survey expressed dissatisfaction with their current PsO treatments, finding them bothersome (topical: 75%, oral: 66%, injectable: 84%).1 Among patients who received conventional oral medications in the UPLIFT survey, up to 57% of them discontinued their treatment, which can mostly be attributed to lack of effectiveness (28%), loss of treatment effectiveness over time (22%), adverse events on other organs (19%) and lack of tolerability.1 As such, Dr. Stein Gold emphasized the crucial need to introduce safer and more effective treatment options to address the challenges faced by PsO patients.
TYK2: A central link in the IL-23/IL-17 pathway of PsO pathogenesis
Recent research has identified TYK2, a component of the JAK-signal transducer and activator of transcription (JAK-STAT) pathway, as a promising therapeutic target for PsO.2 TYK2 plays a vital role in the type I interferon (IFN) and IL-23/Th17 signaling pathway, which is considered the core mechanism underlying the formation of psoriatic plaques.2,3 Dr. Stein Gold remarked, “TYK2 serves as the central link in the IL-23/IL-17 axis, transmitting pro-inflammatory signals in the pathway.”
In PsO, TYK2 serves as a mediator for type 1 IFN signaling and activates STAT1 and STAT2, which induces significant inflammatory effects such as reducing the function of regulatory T cells, activating B cells and polarizing Th17 cells.2,3 In the IL-23/Th17 signalling pathway, TYK2 also mediates the signaling of IL-23, resulting in the amplification of IL-17 responses.2 Such elevated IL-17 responses would further accelerate keratinocyte hyperproliferation and contribute to the rapid development of psoriatic plaques.2
Deucravacitinib exhibits notable performance in treating PsO patients via TYK2 inhibition
Deucravacitinib is a first-in-class oral TYK2 inhibitor that selectively binds to the regulatory domain of TYK2 and limits the downstream pro-inflammatory signaling of the IL-23/Th17 pathway.4 In vitro whole blood assays have affirmed deucravacitinib’s high selectivity towards TYK2.7 At clinically relevant concentrations (6mg once daily [QD]), deucravacitinib achieves a daily average TYK2 activity inhibition of ≥50% whilst having minimal effects on JAK 1/3 and JAK 2/2 activity inhibition (a daily average percent inhibition of ≤1%).7
The significant clinical efficacy and tolerability of deucravacitinib in treating moderate-to-severe PsO compared to placebo have been demonstrated in the 52-week, double-blinded, placebo-controlled phase 3 POETYK PSO-1 and PSO-2 trials.8-10 In both trials, patients with moderate-to-severe PsO (defined as static Physician's Global Assessment [sPGA] ≥3, Psoriasis Area and Severity Index [PASI] ≥12, and body surface area [BSA] involvement ≥10% for ≥6 months before screening) were randomized 2:1 to receive deucravacitinib 6mg QD or placebo for 16 weeks.8,9 At week 16, patients from the placebo arm in both trials switched to deucravacitinib treatment until week 52.8,9
The co-primary endpoints in both studies were the response rates for ≥75% reduction from baseline in PASI (PASI 75) and sPGA of 0 or 1 (sPGA 0/1) at week 16, in which deucravacitinib was associated with significantly higher response rates compared to placebo in the POETYK PSO-1 (PASI 75 = 58.4% vs. 12.7%; p<0.0001, sPGA 0/1 = 53.6% vs. 7.2%; p<0.0001) and POETYK PSO-2 (PASI 75 = 53.0% vs. 9.4%; p<0.0001, sPGA 0/1 = 49.5% vs. 8.6%; p<0.0001) trials.8,9 In addition, deucravacitinib was also associated with a higher achievement rate of PASI 90 among PsO patients at week 16 of the POETYK PSO-1 (35.5% vs. 4.2%; p<0.0001) and the POETYK PSO-2 (27.0% vs. 2.7%; p<0.0001) trials compared to placebo.8,9
Regarding safety, the pooled safety data of the two trials indicated the overall exposure-adjusted incidence rates (EAIR) of adverse events (AEs) and serious AEs in the deucravacitinib arm were comparable to those in the placebo arm at week 52 (AEs: 229.2 vs. 217.4/100 person-years, serious AEs: 5.7 vs. 5.7/100 person-years), with the most commonly observed (≥2%) AEs in the deucravacitinib arm being nasopharyngitis (16.8%), upper respiratory tract infection (9.1%) and headache (5.9%).10 Treatment discontinuation rates due to AEs at week 52 were also relatively lower in the deucravacitinib arm (3.5% vs. 3.2%).10
Dr. Stein Gold also highlighted the relatively low initiation requirements of deucravacitinib, where no dose titration or adjustments for patients with renal or hepatic impairment are needed. In addition, she emphasized that no significant drug-drug interactions were observed, which can be crucial for patients with multiple pre-existing treatments. Coupled with a relatively simple treatment regimen (6mg QD with no laboratory monitoring required), Dr. Stein Gold believed that the practical advantages of oral deucravacitinib treatment could further promote the success of disease remission in PsO patients.
Efficacy of deucravacitinib demonstrated in high-impact areas
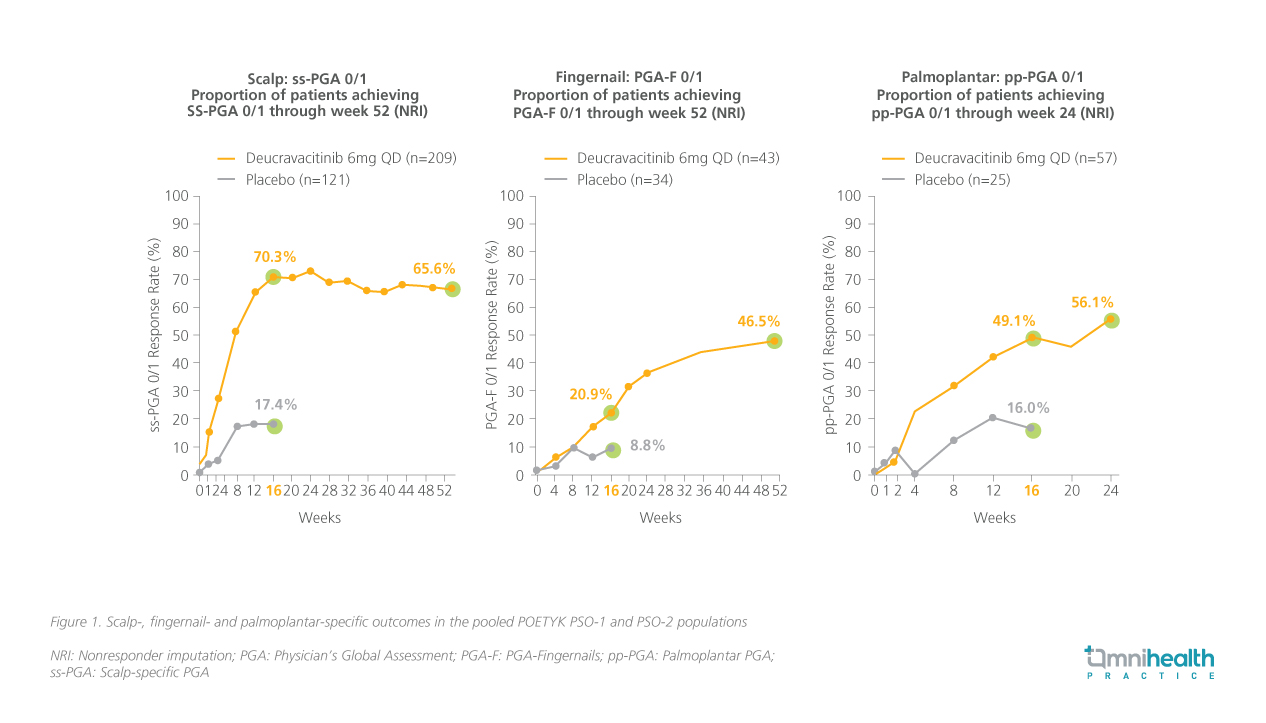
Aside from its noteworthy overall clinical performance, deucravacitinib has also been shown to be capable of alleviating PsO in difficult-to-treat areas in the POETYK PSO-1 and PSO-2 trials.11,12 Subsequent pooled analyses of the two trials were conducted to evaluate the efficacy of deucravacitinib in treating PsO manifestations in high-impact areas, such as the scalp, fingernails, palms and soles.11,12 Patients with moderate-to-severe scalp PsO (scalp-specific Physician's Global Assessment [ss-PGA] ≥3), fingernail PsO (PGA-Fingernails [PGA-F] ≥3) and palmoplantar PsO (palmoplantar PGA [pp-PGA] ≥3) at baseline were enrolled into the analyses.11,12
The findings suggested that deucravacitinib consistently reduced the burden of disease in these specific areas, offering significant relief.11,12 At week 16, patients receiving deucravacitinib demonstrated significantly higher response rates in achieving ss-PGA 0/1 (70.3% vs. 17.4%; p<0.0001), PGA-F 0/1 (20.9% vs. 8.8%), and pp-PGA 0/1 (49.1% vs. 16.0%; p<0.0001) compared to those on placebo.11 The response rates for all three assessments continued to increase after patients in the placebo arm crossed over to deucravacitinib treatment following week 16 (figure 1).11,12
Consistent clinical efficacy and safety of deucravacitinib over 3 years
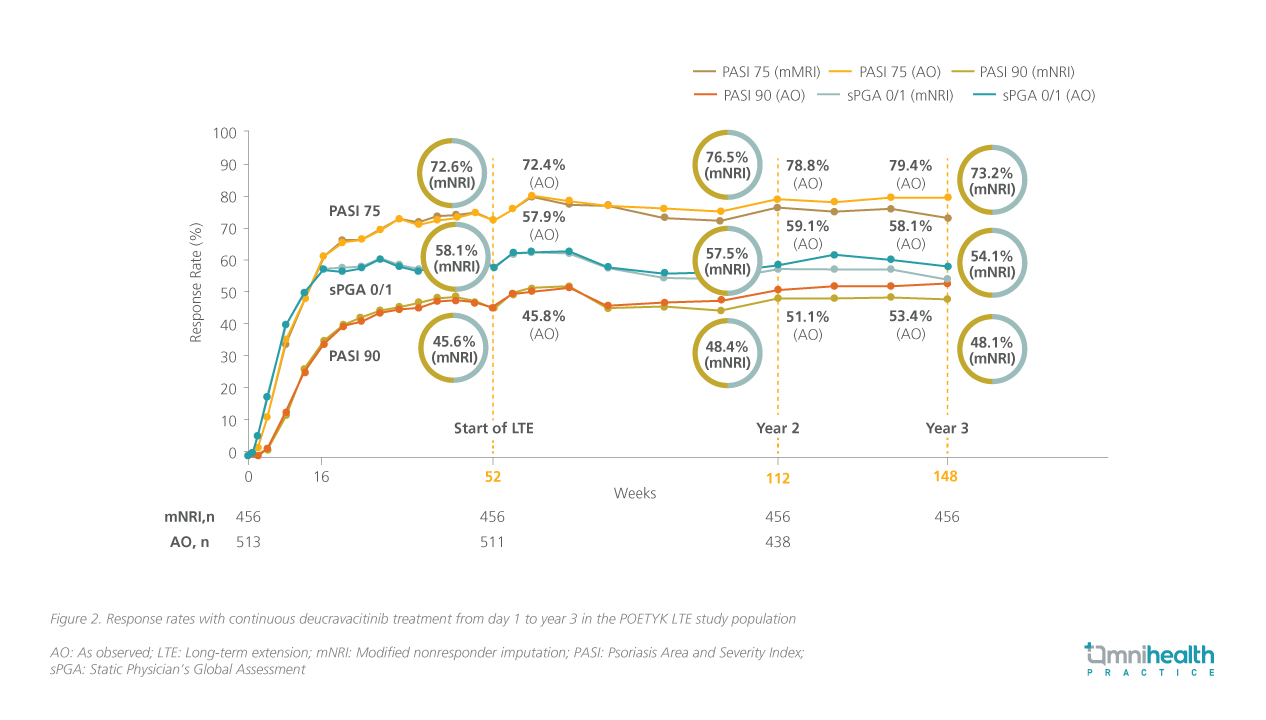
Further investigations based on the two phase 3 trials provided evidence for the sustained long-term clinical efficacy and tolerability of deucravacitinib for up to 3 years.13 In the POETYK PSO-1 and PSO-2 trials, patients who achieved at least PASI 75 response at week 16 (primary endpoint) or week 24 (peak response) were eligible to enter the POETYK long-term extension (LTE) trial, where they received open-label deucravacitinib 6mg QD.13
The proportion of patients who maintained PASI 75, PASI 90 and sPGA 0/1 responses remained consistent from week 52 (start of the POETYK LTE trial) through week 148 (figure 2).13 In terms of safety, the cumulative EAIR of AEs and serious AEs remained manageable at year 2 (AEs: 154.4/100 person-years; serious AEs: 6.1/100 person-years) and year 3 (AEs: 144.8/100 person-years; serious AEs: 5.5/100 person-years).13 Treatment discontinuation rates due to AEs also remained manageable throughout the POETYK LTE trial, with rates of 4.5% at year 2 and 5.1% at year 3.13
Conclusion
In conclusion, deucravacitinib is a first-in-class oral TYK2 inhibitor that effectively inhibits PsO plaque formation by binding selectively to the TYK2 protein in the IL-23/Th17 pathway.6,7 The POETYK PSO-1 and PSO-2 trials have demonstrated impressive efficacy and tolerability of deucravacitinib in treating moderate-to-severe PsO, facilitating significantly higher achievement rates of PASI 75, PASI 90 and sPGA 0/1 responses.8-10 Subsequent analyses have illustrated deucravacitinib’s efficacy in treating PsO manifestations in high-impact areas, while the open-label POETYK LTE trial substantiated deucravacitinib’s clinical performance and safety profile being sustained for more than 3 years.11-13 Considering its simple treatment regimen and consistent efficacy and safety outcomes, Dr. Stein Gold concluded that deucravacitinib is a reliable treatment option for aiding PsO patients in achieving remission.

