CONFERENCE UPDATE: AAN 2024
Updated long-term efficacy of satralizumab in relapse prevention for AQP4-IgG+ NMOSD patients in the SAkuraMoon study
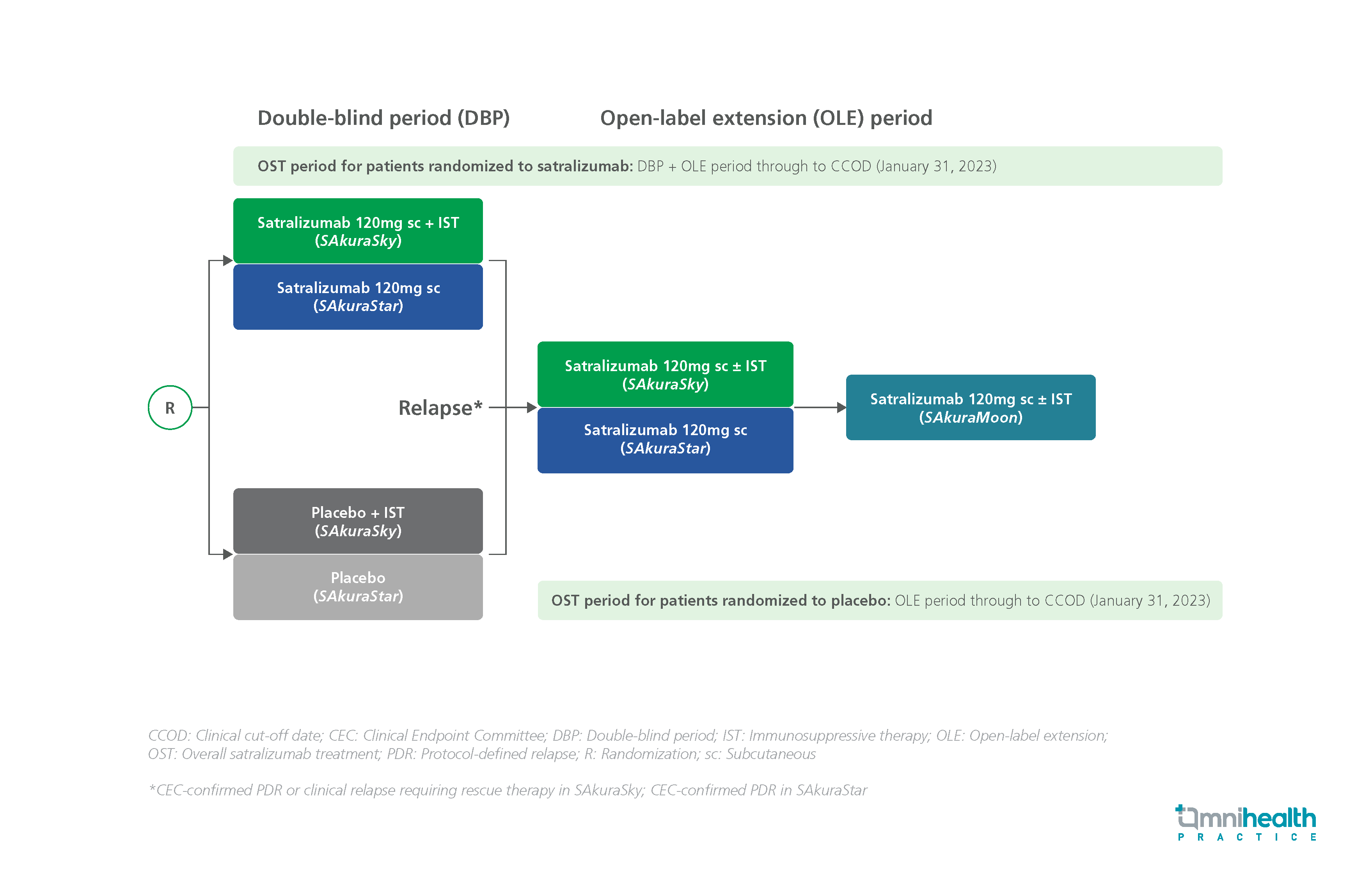
STUDY DESIGN
Satralizumab is a monoclonal recycling antibody that targets the interleukin-6 receptor (IL-6R).1 Two randomized, placebo-controlled, phase 3 clinical trials, SakuraSky and SAkuraStar, had demonstrated the significant reductive effect and favorable safety and tolerability profile of 120mg satralizumab subcutaneous (SC) administration in the risk of relapse in patients with aquaporin-4 immunoglobulin G seropositive (AQP4-IgG+) neuromyelitis optica spectrum disorder (NMOSD).1
This open-label extension (OLE) SAkuraMoon study aimed to assess the long-term efficacy of satralizumab in patients with AQP4-IgG+ NMOSD.1 Participants who completed SAkuraSky and SAkuraStar, were invited to the OLE period where they received 120mg satralizumab SC every 4 weeks, with or without concomitant immunosuppressive therapy (IST).1 There were 111 patients with AQP4-IgG+ NMOSD in this study.1 In this female-dominant (90.1%) study population, patients averaging 43.2 years of age were treated with satralizumab for a median duration of 5.9 years.1 Approximately one-third of the patients were from Asia and the remaining were from Europe or other regions.1
Efficacy analyses were conducted based on data collected in the overall satralizumab treatment (OST) period, from patients’ first dose of satralizumab in the double-blind period (DBP) or OLE period to the clinical cut-off date of January 31, 2023.1 Assessments of efficacy included time to the first protocol-defined relapses (iPDRs), severe relapse (iPDR associated with a ≥2-point increase in Expanded Disability Status Scale [EDSS]), sustained EDSS worsening (if baseline score=0: increase ≥2 points; if baseline= 1-5: increase ≥1 point; if baseline ≥5.5: increase ≥0.5 points) and annualized iPDR rate (ARR).1
FINDINGS
|
Efficacy outcomes: |
|
|
Safety: |
|
“Efficacy of satralizumab ± IST in patients with AQP4-IgG+ NMOSD observed in the DBPs was sustained with long-term treatment”
Dr. Jeffery L. Bennett
University of Colorado School of Medicine,
Aurora, United States

