CONFERENCE UPDATE: AAN 2024
Long-term efficacy and safety of ofatumumab treatment for up to 6 years demonstrated in patients with RMS
Ofatumumab is a fully human monoclonal antibody (mAb) against CD20.1 Monthly subcutaneous injections of ofatumumab 20mg have previously been approved for the treatment of relapsing multiple sclerosis (RMS) in adult patients.1 Previously in the phase 3 ASCLEPIOS I/II trials, ofatumumab led to a greater reduction of the clinical and magnetic resonance imaging (MRI) disease activity in people with RMS (pwRMS) up to 30 months compared with teriflunomide.1 Additionally, results from the open-label extension (OLE) study, ALITHIOS, substantiated the sustained efficacy of ofatumumab in pwRMS for up to 5 years.1 In the captioned analysis, the longer-term safety and efficacy of ofatumumab treatment for up to 6 years in pwRMS were evaluated to further elucidate the benefit-risk profile of ofatumumab in pwRMS.1
The efficacy analyses included data from all participants (n=1,882) randomized to receive ofatumumab (n=946) or teriflunomide (n=936) in the ASCLEPIOS I/II trials.1 The safety analyses included data from all participants who received at least one dose of ofatumumab in the ASCLEPIOS I/II, APOLITOS, APLIOS or ALITHIOS trials (n=1,969).1 Participants treated with teriflunomide in ASCLEPIOS I/II were switched to ofatumumab (TER-OMB group), while those assigned ofatumumab in the initial trial continued to receive ofatumumab in the ALITHIOS OLE (OMB-OMB group).1
The efficacy outcomes assessed included the annualized relapse rate (ARR), mean number of gadolinium-enhancing (Gd+) T1 lesions per scan, number of new or enlarging T2 (neT2) lesions per year, 6-month confirmed disability worsening (6mCDW) and no evidence of disease activity (NEDA-3) which was defined by having none of the above outcomes.1 Safety outcomes included the overall safety profile, serious infections, malignancies and laboratory parameters (immunoglobulin [Ig] G and IgM levels), lymphocyte and neutrophil counts and malignancies.1
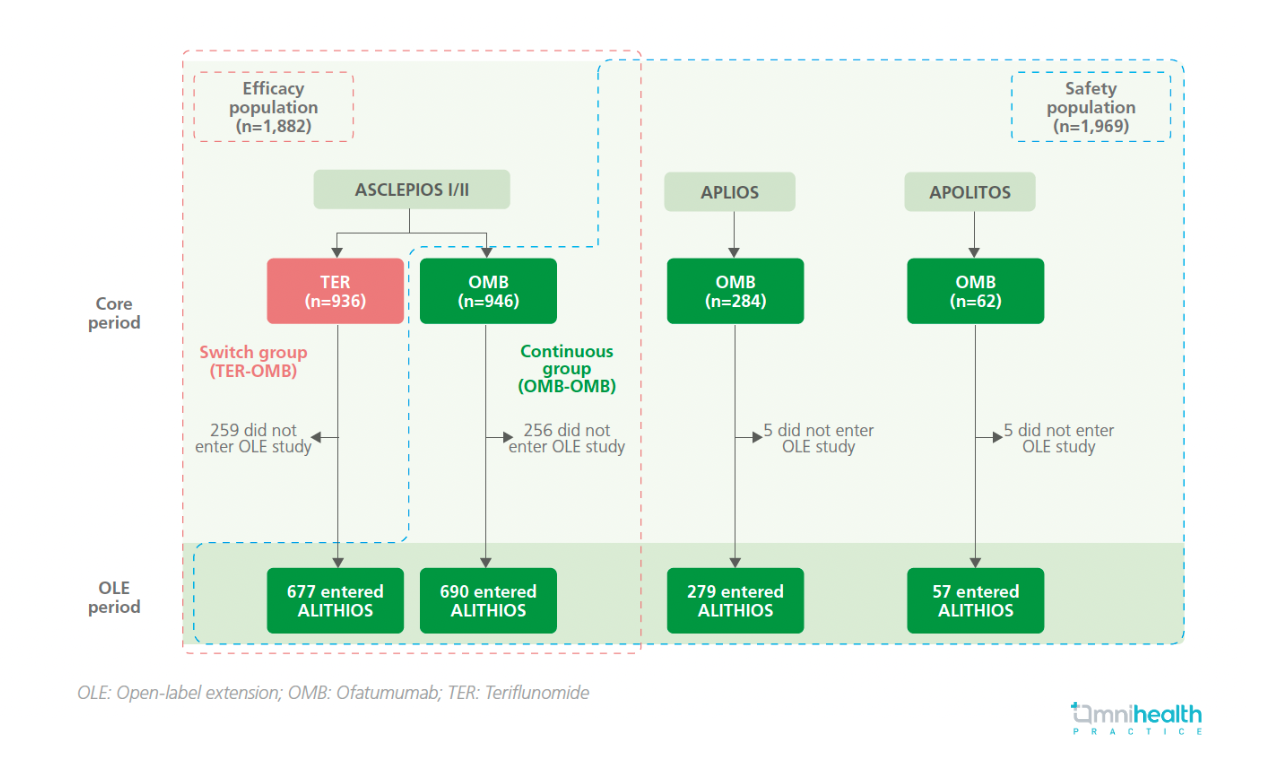 FINDINGS
FINDINGS
|
Efficacy outcomes: |
|
|
|
|
|
|
|
Safety: |
|
|
“These results support the long-term, favorable benefit-risk profile of ofatumumab treatment (up to 6 years) and reinforce the benefit of early ofatumumab initiation in pwRMS”
Dr. Tuan Vu
College of Medicine,
University of South Florida Morsani,
Florida, United States

