CASE REVIEW
Case sharing: Improving treatment outcome in MDD patients with timely brexpiprazole augmentation
Treatment-resistant depression (TRD) is defined as treatment failure following ≥2 trials of antidepressant therapy (ADT) at adequate doses, duration and adherence.1 Around 30% of patients with major depressive disorder (MDD) become resistant to conventional treatments.2 TRD is independently associated with elevated morbidity and mortality compared with other MDD patients.1 Augmentation with atypical antipsychotics (AAPs) like brexpiprazole provides an important option for these patients.1 In an interview with Omnihealth Practice, Dr. Lai, Chi-Lun illustrated the case for early AAP augmentation, particularly for patients with more severe disease. He shared the story of a high-functioning young lady with a very disabling form of MDD. Upon receiving timely augmentation with brexpiprazole early in the patient journey, she achieved rapid functional recovery and was able to catch up with the demands of her diligent lifestyle.
Background
Functional impairment brought on by MDD inevitably leads to a deterioration in the patient’s quality of life (QoL).3 Improving their QoL is essential in minimizing the disability experienced by these patients.3 The burden of MDD is even higher in patients with TRD.1 Patients inadequately managed on ADT experience prolonged loss of QoL and functioning, imposing a significant burden not only on themselves but society as a whole and incurring additional costs associated with work productivity loss and increased utilization of healthcare resources.4
Despite the extensive arsenal of ADT, up to 50% of all MDD patients fail to achieve an adequate response.1 Even among patients who achieve partial remission, residual depressive symptoms are associated with a worse QoL, subsequently creating a larger burden.3 Recent studies have identified a clear and present need for more rapid advancement to the next line of study if the initial lines of therapy do not produce sufficient response.5 The National Institute for Health and Care Excellence (NICE) guidelines recommend an initial strategy of switching between different ADTs followed by augmentation with AAPs as plausible methods to help these patients.6 Previous large-scale meta-analyses have shown that adjunctive treatment with AAP increased the odds of response and remission to ADT by 1.61 times and 1.77 times respectively.4 However, traditional AAPs often come with sequelae of activating, sedating and metabolic issues, leaving much to be desired.4 Therefore, an AAP option like brexpiprazole, which is both effective and well-tolerated, is required for patients to benefit from their ADT regimen.4
Dr. Lai stressed that MDD may be very debilitating for the patients. Symptoms, like reduced energy level and cognitive impairment, may affect their lives at work, at school, or even for homemakers when they cannot fulfill their functions. However, if the MDD episode can be remitted early, the impact on their functioning can be minimized. In the following case sharing, Dr. Lai demonstrated how a decisive call to initiate early brexpiprazole augmentation helped a young lady get her life back on track.
Case sharing
The patient is a 22-year-old girl studying law in the United Kingdom (UK) with no relevant history of psychiatric illness, alcohol, or drug use. Her education was put on hiatus due to her depressive symptoms and she took leave to return to Hong Kong seeking psychiatric help in June of 2023. She reported pervasive low mood, decreased energy level, loss of interest, and difficulty concentrating on her studies. She also presented with a lot of negative self-cognition and blamed herself for her unsatisfactory academic performance, triggering internalized guilt of being a burden to her family.
The diagnosis of MDD was made promptly after ruling out the possibility of thyroid function abnormality. First-line treatment of sertraline 50mg daily (QD) was initiated and was up-titrated to 75mg (QD) after 2 weeks of follow-up. However, the response was inadequate with marginal improvement in mood. QoL and functioning did not improve with persisting negative cognition and issues with concentration. Considering that the new semester begins in September, the patient expressed that she did not wish to wait 2 additional weeks for an uncertain chance of improvement with the ADT switching strategy, AAP augmentation was chosen instead to expedite improvement. As a young woman who wished to continue her academic pursuits, both sedation and weight gain were major considerations, thus brexpiprazole was chosen as the appropriate augmentation option. Brexpiprazole 0.5mg (QD) was added on top of the sertraline regimen and was up titrated to 1mg (QD) in early July.
Remission, as defined by the ability to catch up with her studies as well as lower incidences of negative cognition, was achieved shortly in mid-August 2023. Dr. Lai remarked that the patient is smart and high-functioning and that she knows how her studies are going. After the initiation of brexpiprazole, she reported major mood improvement, and the treatment has restored her ability to concentrate, even managing to pick up the materials she missed out on last semester. Throughout brexpiprazole augmentation, she has reported no instances of extrapyramidal symptoms (EPS) or akathisia. She continued her follow-up every 2 weeks until she went back to the UK for the new semester in early September.
Discussion
Brexpiprazole: A standout option for mood improvement and safety in mind
Dr. Lai claimed that this case is representative of many young patients where both the efficacy and adverse effect (AE) profiles are important in the selection of augmentation, as it may affect the patient’s adherence to the treatment. He reminded us that there are many drugs available for augmentation, and it is important to consider the AE profile of specific drugs and tailor treatment to each patient.
In a pooled analysis of 4 randomized, double-blinded trials, patients (n=1,853) with MDD and inadequate response to ADTs were treated with adjunctive brexpiprazole 1-3mg*.4 Using numbers needed to harm (NNH) as the basis of comparison, brexpiprazole showed to be less activating compared with aripiprazole and less sedating when compared with quetiapine extended-release and olanzapine-fluoxetine combinations.4 The rates of discontinuation due to treatment-emergent adverse effects (TEAEs) over 6 weeks of treatment were low at 2.2%.4 There were low incidences of akathisia (8.0%) and sedation (0.2%).4 No AEs regarding metabolic parameters or prolactin levels were reported with only mild mean weight gain of 1.4kg over 6 weeks (table 1).4
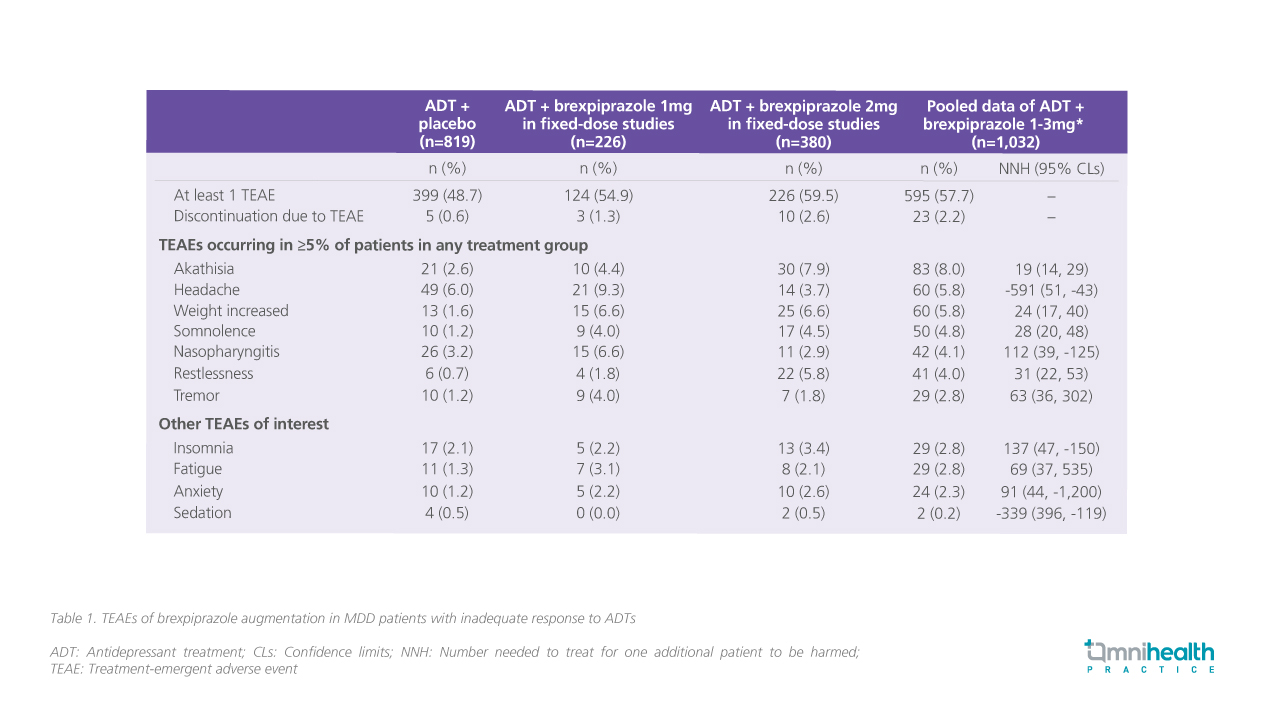
Dr. Lai attributed the safety profile of brexpiprazole to the concurrent and selective modulation effect on both the serotonin and dopamine receptors. He highlighted that with partial agonism at the dopamine receptors and less histaminergic effects at the histamine type 2 (H2) receptor, brexpiprazole offers a more favorable AE profile in terms of weight gain, metabolic parameters, and sedation as compared with other AAPs.
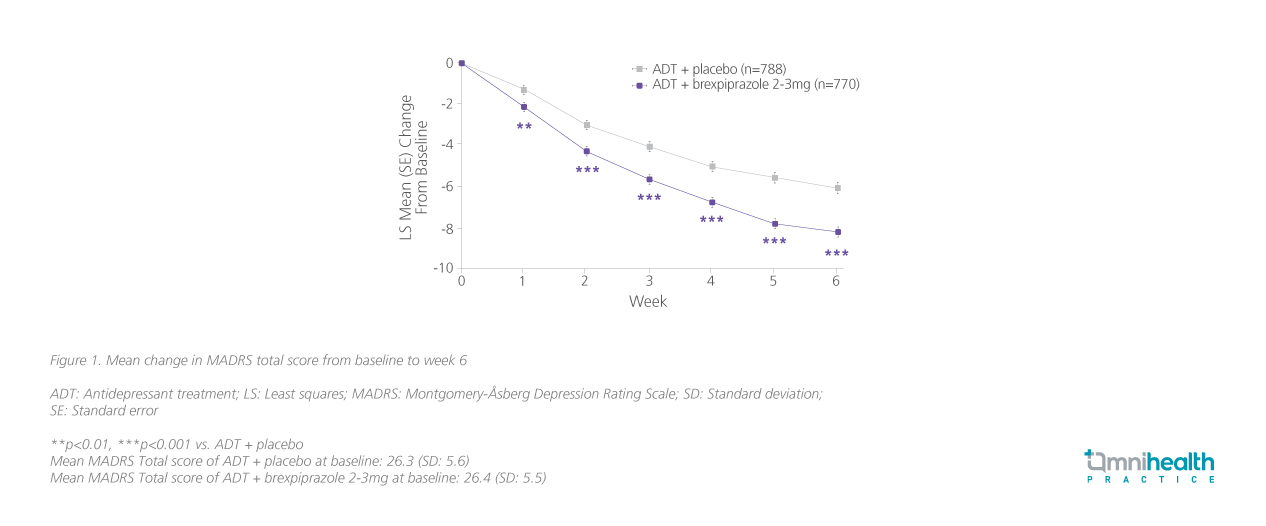
In the same study, improvement in the Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline was higher with brexpiprazole augmentation vs. ADT + placebo only [Least squares mean (LSM) difference=-2.15; CL: -2.82 to -1.48; p<0.0001] after only 6 weeks of treatment.4 Importantly, significant improvement vs. ADT + placebo occurred after 1 week of brexpiprazole augmentation (figure 1).4
Rapid improvement in life-engagement with brexpiprazole augmentation
Dr. Lai was very impressed with how quickly the patient was able to reengage with her studies following brexpiprazole augmentation. He remarked that besides clinical questionnaires like the Patient Health Questionnaire-9 (PHQ-9), life-engagement criteria like the restoration of baseline functioning and the resumption of responsibilities are important markers of clinical response.7
In a retrospective study conducted among adult patients with MDD, measures of life-engagement score were derived and quantified based on real-world information collected from clinical labels identified from the mental state examination (MSE) in actual psychiatric practices.7 A total of 54 labels were identified and sorted into 4 distinct domains of emotional, physical, social, and cognitive items (table 2).7

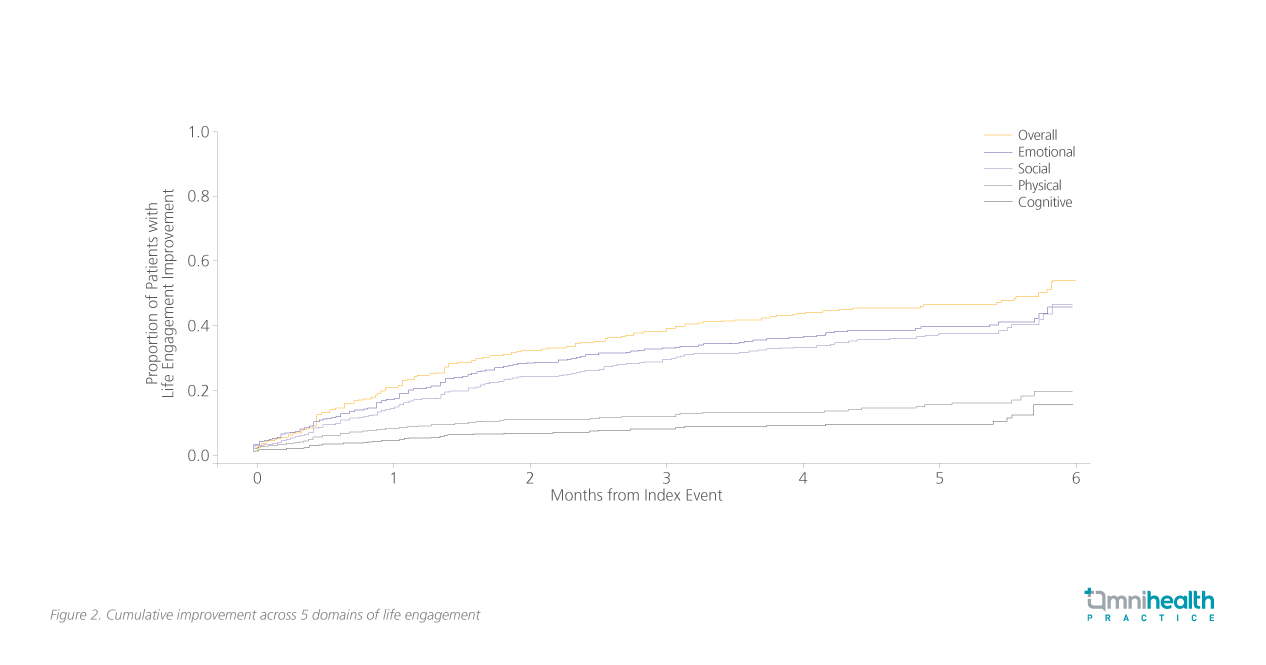
The scale was then used to evaluate the improvement of adult MDD patients (n=624) who received brexpiprazole as adjunctive therapy to ADT over 6 months.7 A statistically significant 20.6% improvement in life-engagement was observed as early as after 1 month of treatment.7 The rate of improvement continued to increase across the 3-month and 6-month mark at 37.9% and 53.9% respectively.7 Within the first 6 months, the improvements were more evident across the emotional and social domains at 44.5% (Survival probability=0.56; 95% CI: 0.48-0.62) and 45.6% (Survival probability=0.54; 95% CI: 0.45-0.63) respectively; less profound improvements were observed across the physical and cognitive domains at 19% (Survival probability=0.81; 95% CI: 0.75-0.86) and 14% (Survival probability=0.86; 95% CI: 0.79-0.91) respectively as presented in figure 2.7 The rapid response achieved in terms of life functioning is echoed in the case of early brexpiprazole augmentation.
Conclusion
Dr. Lai concluded that when the initial response to ADT appears inadequate, we can consider augmentation earlier in the treatment paradigm to minimize the adverse impact on the patient’s life functioning. Brexpiprazole has demonstrated its effectiveness in allowing MDD patients to reengage with their lives all the while with a tolerable AE profile.4 The young woman in the case sharing initially thought she would never recover from her negative cognitions, but with the right medication initiated at the right time, Dr. Lai reassured her that “all the bad shall pass,” allowing her to effectively reengage her life and regain the confidence she had lost.
*Note that in Hong Kong, brexpiprazole is only indicated as an adjunctive therapy to ADT in patients with MDD at a starting dose of 0.5-1mg per day, up to a maximum of 2mg per day. Maximum dose in adjunctive treatment of 3mg has not been approved as of Feb 2024.

