CONFERENCE UPDATE: AAAAI 2024
Interim analysis of the real-world CHOPIN study shows reduction in HAE attacks and improvement in PROs with lanadelumab
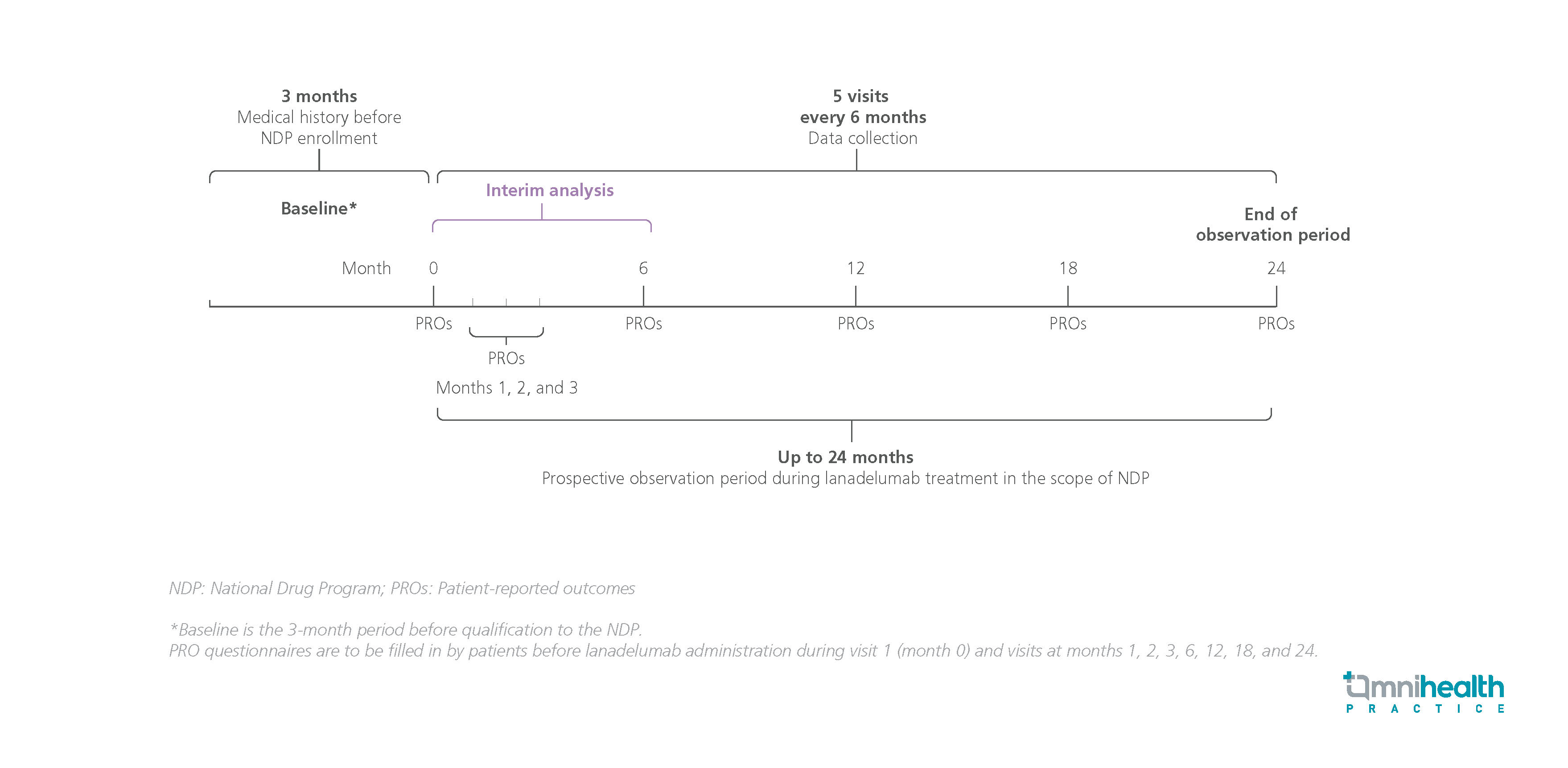
STUDY DESIGN
Hereditary angioedema (HAE) is a rare genetic disease caused by a deficiency of the C1-inhibitor protein which causes uncontrolled plasma kallikrein activity.1 HAE is characterized by recurrent swelling episodes that affect the abdomen, larynx or extremities.1 It is also associated with laryngeal edema attacks which constrict the respiratory tract and may become a life-threatening emergency.1 Based on global guidelines for HAE management, lanadelumab is recommended as first-line long-term prophylactic HAE management.1 It is a fully monoclonal antibody that inhibits active plasma kallikrein, and has been shown to reduce the frequency of HAE attacks and improve patient-reported health-related quality of life (QoL) in the phase 3 HELP trial.1 However, data on the efficacy of lanadelumab in HAE patients in the real world remain limited.1
CHOPIN is a noninterventional, prospective, multicenter real-world study conducted to assess the effectiveness of lanadelumab in HAE patients naïve to prophylaxis.1 The study included patients with HAE aged ≥12 years who qualified for treatment in the Polish National Drug Program (NDP) and consented to participate in the study.1 Data from the 6-month interim analysis of 16 patients were represented.1
The primary endpoints were the change in the total number of HAE attacks from baseline to 3 months after lanadelumab initiation, the proportion of HAE attacks requiring rescue treatment and the characteristics of HAE attacks.1 Secondary endpoints included the change in the Work Productivity and Activity Impairment Questionnaire: General Health (WPAI:GH) and Angioedema Quality of Life (AE-QoL) scores from baseline to 6 months after lanadelumab initiation.1 Other exploratory endpoints were the change in the Angioedema Control Test (AECT) score from baseline to 6 months and the incidence, type, severity, seriousness, and treatment-relatedness of adverse events (AEs).1
FINDINGS
| Primary endpoints: |
|
|
|
|
| Secondary endpoints: |
|
|
|
|
| Safety: |
|
“These findings are consistent with those from pivotal and real-world studies supporting the use of lanadelumab as first-line prophylactic treatment in patients with HAE"
Dr. Aleksandra Kucharczyk
Department of Internal Diseases,
Pneumonology, Allergology, Clinical Immunology,
Military Institute of Medicine, Warsaw, Poland

