CONFERENCE UPDATE: ASH 2023
Mosunetuzumab demonstrates consistent efficacy in patients with R/R follicular lymphoma after ≥2 prior treatments: 3-year follow-up from a pivotal phase 2 study
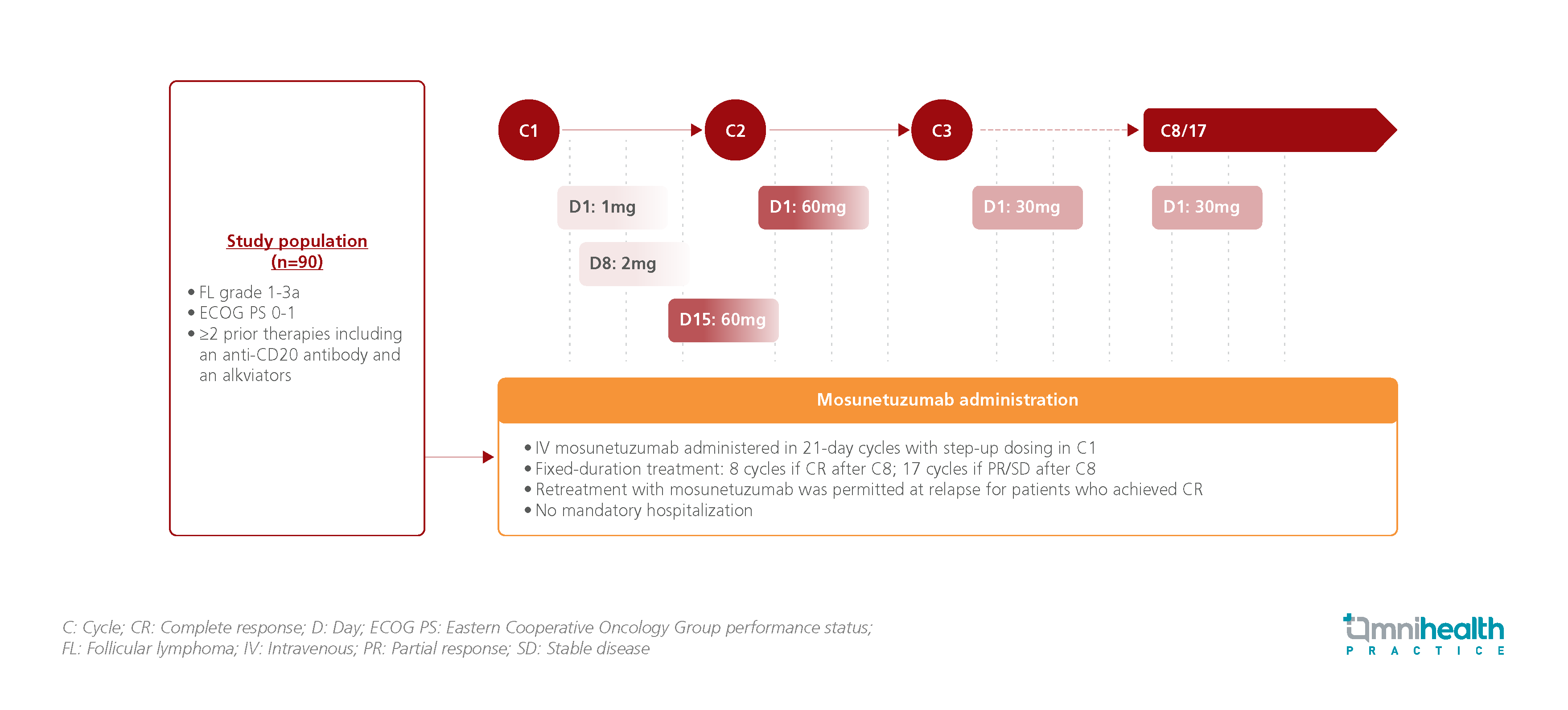
STUDY DESIGN
Mosunetuzumab is a T-cell-engaging bispecific antibody that redirects T cells to eliminate malignant B cells.1 It has been approved for the treatment of relapsed and/or refractory (R/R) follicular lymphoma (FL) after ≥2 prior systemic treatments.1 Previous analysis of the pivotal phase II study at a median follow-up of 18.3 months has shown mosunetuzumab’s high response rates with durable responses in heavily pre-treated patients with R/R FL.1 A subsequent analysis with a median follow-up of 37.4 months was conducted to provide updated results from the study.1
The pivotal phase II study was a single-arm study that investigated the efficacy and safety of mosunetuzumab among R/R FL patients pre-treated with ≥2 prior treatments.1 90 patients with R/R FL and ≥2 prior therapies, including an anti-CD20 antibody and an alkylator, were enrolled and were administered mosunetuzumab intravenously in 21-day cycles.1 Dosing was stepped up from 1mg to 60mg in cycle 1.1 All patients received 8 cycles of treatment, with those who showed partial response (PR) or stable disease (SD) continuing treatment until cycle 17.1 Retreatment was permitted at relapse for patients who achieved complete response (CR).1
The endpoints assessed in this update analysis included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.1
|
Efficacy: |
|
|
Safety: |
|
“In heavily pre-treated patients with R/R FL, fixed-duration mosunetuzumab is well-tolerated and can achieve long-lasting remissions.”
Dr. Stephen Schuster
Lymphoma Program,
Abramson Cancer Center,
University of Pennsylvania,
Philadelphia, PA

