CONFERENCE UPDATE: ASH 2023
Ruxolitinib exhibits sustained efficacy compared to best available treatment in patients with chronic GvHD: 3-year final analysis of the REACH3 study
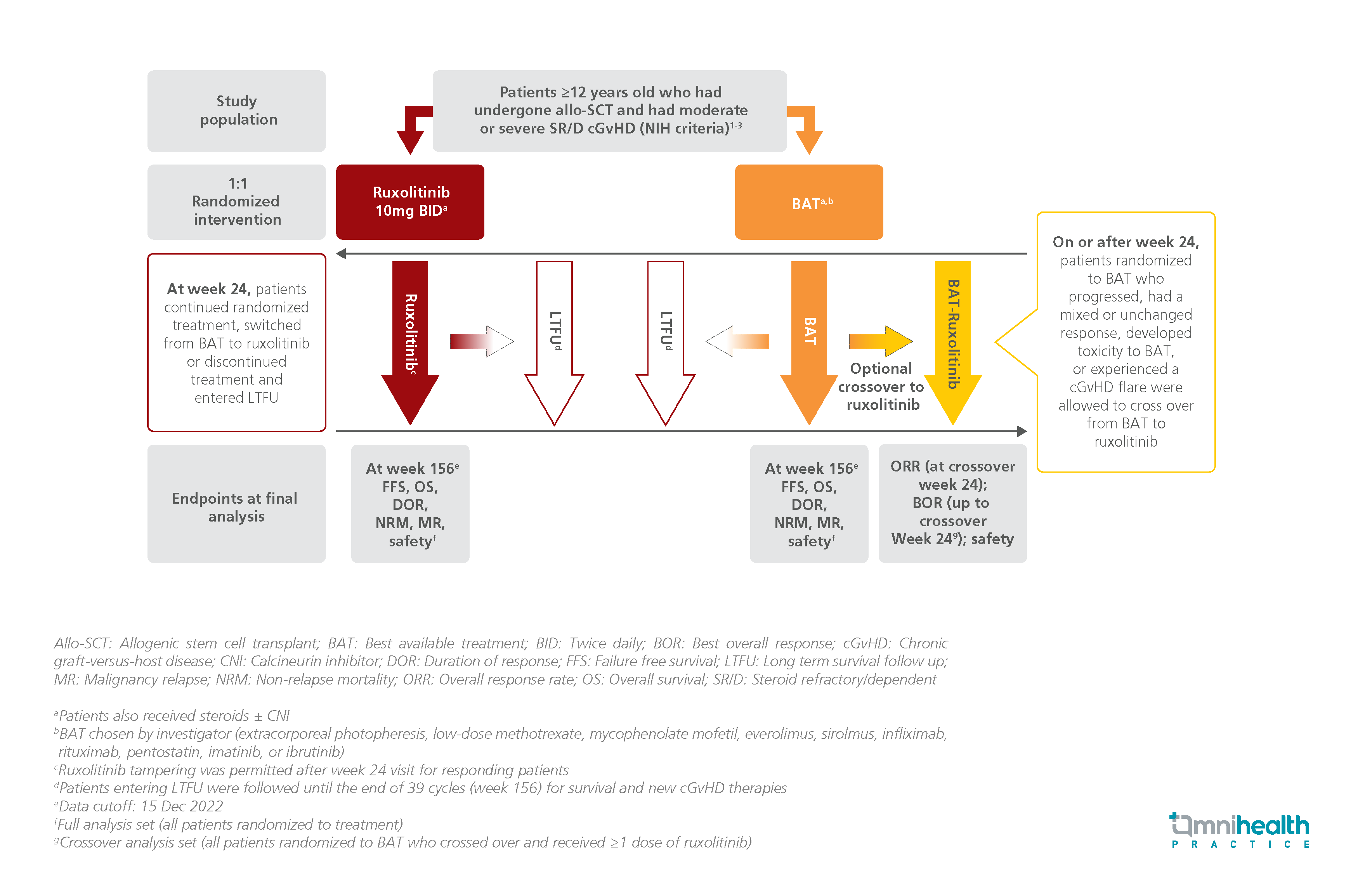
STUDY DESIGN
Graft-versus-host disease (GvHD) is a life-threatening complication of allogeneic stem cell transplant (allo-SCT) that occurs in half of the patients who undergo the procedure.1 Although corticosteroids are the first-line treatment options for GvHD, a majority of patients become refractory towards them.1 Ruxolitinib is a Janus kinase (JK)1/JK2 inhibitor that demonstrated notable efficacy over best available treatment (BAT) in the initial analysis of the REACH3 study, exhibiting significantly higher overall response rate (ORR) at 24 weeks of treatment (49.7% vs. 25.6%; p<0.0001).1
The REACH3 study was a randomized phase 3 trial that assessed the efficacy and safety of ruxolitinib and BAT in patients with GvHD.1 329 patients who were aged ≥12 years and developed moderate or severe steroid-refractory/dependent chronic GvHD (cGvHD) after undergoing allo-SCT were randomized 1:1 to receive either ruxolitinib 10mg twice daily (n=165) or BAT (n=164).1 The primary analysis was conducted at week 24, after which BAT patients were allowed to crossover to ruxolitinib, continue BAT, or discontinue and enter long-term follow-up (LTFU).1 Patients randomized to the ruxolitinib group were also allowed to discontinue and enter LTFU or continue treatment.1 The final analysis was conducted at week 156.1
The efficacy endpoints for this final analysis included failure-free survival (FFS), duration of response (DOR), overall survival (OS), and safety outcomes of both treatment groups at week 156.1 ORR and best overall response (BOR) of BAT patients who crossed over to ruxolitinib after week 24 were also analyzed.1
|
Efficacy: |
|
|
Safety: |
|
“Ruxolitinib provided longer FFS and DOR versus BAT, demonstrating sustained efficacy for patients with steroid-refractory or dependent chronic GvHD with a consistent and manageable safety profile”
Dr. Robert Zeiser
Department of Medicine I,
Faculty of Medicine,
Medical Center,
University of Freiburg,
Freiburg, Germany

