CASE REVIEW
Where clopidogrel fails: The struggle with CYP2C19 polymorphisms in the treatment of neurovascular diseases
Following an initial transient ischemic attack (TIA) or ischemic stroke (IS), patients have an elevated chance of recurrent stroke.1 One integral component of a comprehensive stroke package is the early initiation of dual antiplatelet therapy (DAPT) in non-cardioembolic stroke, which has become standard over the last decade.1 Recent studies revealed that the efficacy of some P2Y12 receptor antagonists like clopidogrel may be severely limited in certain cytochrome P450 (CYP)2C19 phenotypes which are prevalent among Asians.2-4 However, this can be mitigated by switching to another P2Y12 receptor antagonist like ticagrelor which does not require CYP2C19 metabolism.3,4 In an interview with Omnihealth Practice, Dr. Gary Lau and Dr. Michael Tang discussed 2 clinical cases of elderly patients who had either developed an ischemic event despite DAPT or were at very high risk of developing one. After CYP2C19 genotype testing showed both of the patients to be inadequate metabolizers of clopidogrel, they were switched to ticagrelor and have not had another ischemic event since.
Background
Dual antiplatelet therapy: The cornerstone of recurrent stroke prevention
Recurrent stroke is an imminent threat for patients who have experienced an initial ischemic stroke (IS) or transient ischemic attack (TIA).1 This risk is highly variable but it is associated with risk factors such as the presence of ipsilateral large artery atherosclerosis and cardioembolism.5 Generally the estimated risk of recurrence is 2% within the initial 12 hours, and up to 10% within the first 2 weeks after the initial event.1 Therefore, secondary prevention measures are vital during the acute phases of stroke. In the previous decade, 2 landmark trials that included patients with TIA and minor stroke demonstrated the superiority of aspirin/clopidogrel combination when compared with aspirin alone.6,7 This remarkable achievement ushered the paradigm of single antiplatelet therapy (SAPT) into an era of dual antiplatelet therapy (DAPT).1 The recommendation to start DAPT within 24 hours of stroke for this group have patients has since then been enshrined into guideline recommendations of the American Heart Association (AHA) and European Stroke Organization (ESO).8,9
Unveiling the CYP2C19-dependency of clopidogrel and its impact on stroke prevention
Although clopidogrel is commonly used, it has several pharmacological limitations. Up to 40% of the population fail to achieve adequate response to clopidogrel as defined by a relative change of <10% in platelet aggregation inhibition (IPA).2 This is attributed partially to insufficient metabolite generation since only 15% of clopidogrel is available as its active metabolite.2 The delayed onset of clopidogrel may hinder its effectiveness.2 Results from the ALBION trial suggested that a 300mg loading dose of clopidogrel required an estimated 6 hours to achieve maximal IPA.10
Furthermore, the two-step activation of clopidogrel relies on cytochrome P450 (CYP)-dependent pathways, which can manifest as inconsistencies in its pharmacokinetic and pharmacodynamic profile (figure 1).2-4 Depending on the testing method and the definition of response, the nonresponse rate of clopidogrel can range from 4% to 34%.4 In instances where patients were identified with 2 CYP2C19 loss-of-function (LOF) alleles, platelet inhibition of clopidogrel could still be inadequate with a daily dose of 300mg (4 times the standard dose of 75mg daily).4 The interindividual variability of clopidogrel response can be attributed to CYP2C19 polymorphisms. The Pharmacogene Variation Consortium (PharmVar) has defined 39 allele haplotypes which may lead to normal, reduced, increased, and loss of function.11 Individuals who are characterized as intermediate metabolizers (IM) carry 1 CYP2C19 LOF allele, while poor metabolizers (PM) carry 2 CYP2C19 LOF alleles, namely the CYP2C19*2 and CYP2C19*3 genotypes.4 These individuals may exhibit attenuated antiplatelet effects and have a suboptimal response towards clopidogrel treatment which downplays the overall effectiveness of DAPT in the prevention of recurrent stroke.4
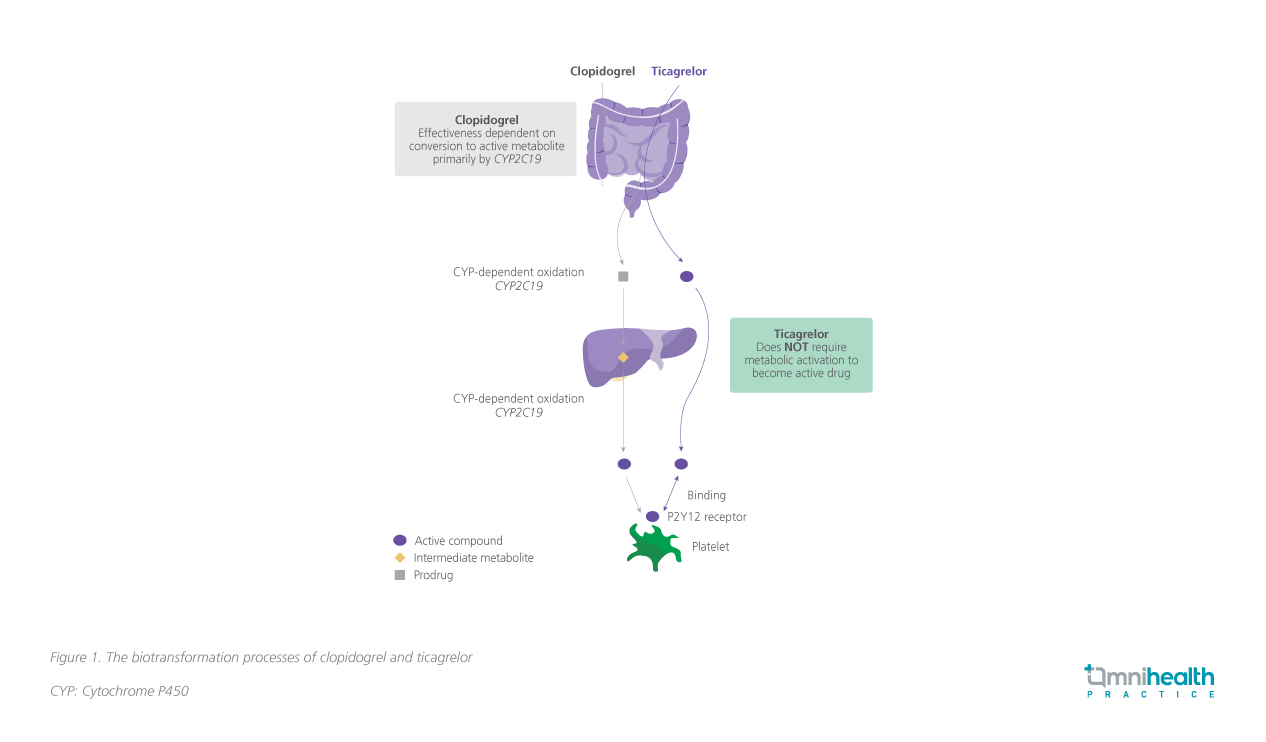
It is preferable that patients who harbor reduced or loss of function CYP2C19 alleles switch to an alternative P2Y12 receptor antagonist.12 Ticagrelor presents as a potent option that is less dependent on CYP2C19 function.13 Moreover, although ticagrelor is responsible for most of the antiplatelet effect, its metabolite is equipotent and contributes to sustained platelet inhibition.3 These advantages, make it a prime candidate for CYP2C19 LOF carriers.
CYP2C19 phenotypes in the local population
Compared to their European and American counterparts, the proportion of CYP2C19 LOF allele carriers is higher among Asian populations.4 The percentages of IM and PM in Asian populations are approximately 45.5% and 12.2% respectively, whereas the percentages of European IM and PM are only 23.0% and 1.8% respectively.4 Dr. Tang remarked there is a trend towards an east-west dichotomy, with western regions showing lower percentages of CYP2C19 LOF alleles. However, global prevalence data are categorized into biogeographical groups to look for broad differences in frequencies. It would be an ecological fallacy to assume local frequencies would resemble group frequencies. Nevertheless, the proportion of IM and PM from exome sequencing data in Hong Kong seems to echo general phenotype frequencies.
CASE REVIEW
A study involving 1,116 Hong Kong Chinese individuals determined that 57.2% of participants exhibited an inferior CYP2C19 metabolizing efficiency with 45.3% and 12.0% of them categorized as IM and PM respectively.14 The data closely resembles the rate of CYP2C19 LOF-allele carriers in the broader Asian population at 59% reported in a systematic review which included studies with clopidogrel-treated patients with stroke or TIA.15 CYP2C19 genotyping data among stroke patients in Queen Mary Hospital also suggests roughly 62% of people in Hong Kong carry a LOF allele.
Despite the concerning prevalence of CYP2C19 LOF carriers and the existence of competent genotype testing technology, Dr. Lau commented that CYP2C19 genotyping testing is only incorporated in selected patient cases, and it is not available for widespread deployment under current local clinical settings. Dr. Tang supplemented that while ideally, all patients should have their CYP2C19 genotype checked prior to DAPT initiation, there are logistical and laboratory considerations that preclude universal rapid testing. Time-course analyses demonstrated that DAPT is most beneficial when it is started within 3-5 days from the index event. Results of universal testing may not be ready at the opportune time when treatment is most relevant. Selection criteria which include high-risk features such as clopidogrel non-responders and a high atherosclerosis burden, is currently used to assess the patient’s urgency for CYP2C19 genotype testing.
Due to the relatively prevalent CYP2C19 LOF carrier phenotypes among the Asian population, the clinical efficacy of clopidogrel-containing DAPT is shackled, leaving patients vulnerable to recurrent stroke.13 Dr. Lau shared 2 clinical cases where he introduced ticagrelor to patients with CYP2C19 LOF alleles for the prevention of recurrent strokes.
Case sharing
Case 1 – Recurrent stroke while on DAPT
A 73-year-old male patient with symptoms of acute headache, vertigo, double vision, and unsteady gait was referred from a private hospital for an abnormal brain magnetic resonance imaging (MRI). The scan showed 2 recent small lacunar infarcts at the left middle cerebellar peduncle, and significant atherosclerosis involving both the intracranial and extracranial arteries, including the bilateral internal carotid arteries (ICA) and middle cerebral artery (MCA) M1 segment, the right anterior cerebral artery (ACA) A1 segment, the left vertebral artery (VA), and the left posterior cerebral artery (PCA) P2 segment. Focal severe stenosis was also observed over the basilar artery. A DAPT regimen of aspirin and clopidogrel (aspirin 320mg QD and clopidogrel 300mg QD, followed by 80mg and 75mg QD respectively) was initiated for this patient.
Unfortunately, 5 days after admission, the patient experienced a new stroke characterized by left facial weakness and numbness, hearing loss over the left ear, and slurred speech, compatible with left lateral pontine syndrome. CYP2C19 genotyping showed the patient to be an intermediate metabolizer (CYP2C19*1/*2), and the DAPT regimen was changed accordingly, where a combination of ticagrelor and aspirin was maintained for 3 months. The patient had not experienced any recurrent strokes after the change.
Case 2 – Carotid Stenting
A 75-year-old male patient with a history of nasopharyngeal carcinoma (NPC) was admitted to the emergency department in December 2020 for acute left sided weakness. Urgent brain computed tomography angiography (CTA) revealed an occlusion of the right cervical ICA and a long segment filling defect in the right MCA M1, indicating the presence of a thrombus. Repeat digital subtraction angiography (DSA) after administering tissue plasminogen activator (tPA) showed residual critical stenosis over the right cervical ICA. After carotid stent deployment and balloon angioplasty, there is complete reperfusion [Thrombolysis in cerebral infarction (TICI) score: 3]. The patient was then discharged with a Modified Rankin Score (mRS) of 0. He was prescribed a 3 months course of DAPT with aspirin and clopidogrel, followed by aspirin monotherapy.
Subsequent monitoring with interval CTA scan during May 2023 showed significant in-stent restenosis and critical ICA stenosis just beyond the existing common carotid artery (CCA)-ICA stent. In addition, a new critical stenosis was found at the opening of the right VA ostium. CYP2C19 genotyping was done this time indicating the presence of homozygous CYP2C19*2 LOF allele, confirming the patient to be a poor CYP2C19 metabolizer. After a new carotid stent was deployed over the CCA-ICA, he was treated with ticagrelor and aspirin for 3 months. The patient remains free of any stroke-related disability as of September 2023.
Discussion
Ticagrelor: A promising P2Y12 receptor antagonist for patients with abnormal CYP2C19 function
DAPT with clopidogrel may be less effective in preventing recurrent strokes in patients with CYP2C19 LOF alleles, leading to disastrous outcomes exemplified by the above case. In this population, agents unaffected by CYP2C19 such as ticagrelor are superior to clopidogrel as demonstrated in the CHANCE-2 study.13
When compared to clopidogrel, ticagrelor was associated with a 23% risk-reduction (HR=0.77; 95% CI: 0.64-0.94; p=0.008) in stroke recurrence within 90 days of the initial ischemic event in the CHANCE-2 study (figure 2).13 CHANCE-2 was a randomized, double-blinded, placebo-controlled study involving 6,412 Chinese patients with CYP2C19 LOF alleles who had a minor ischemic stroke or a recent TIA.13 In addition to aspirin, patients were randomized to receive either 180mg ticagrelor loading followed by 90mg twice daily (BD) for 90 days (n=3,205) or 300mg clopidogrel loading followed by 75mg once daily (QD) for 90 days (n=3,207) within 24 hours of symptom onset.13
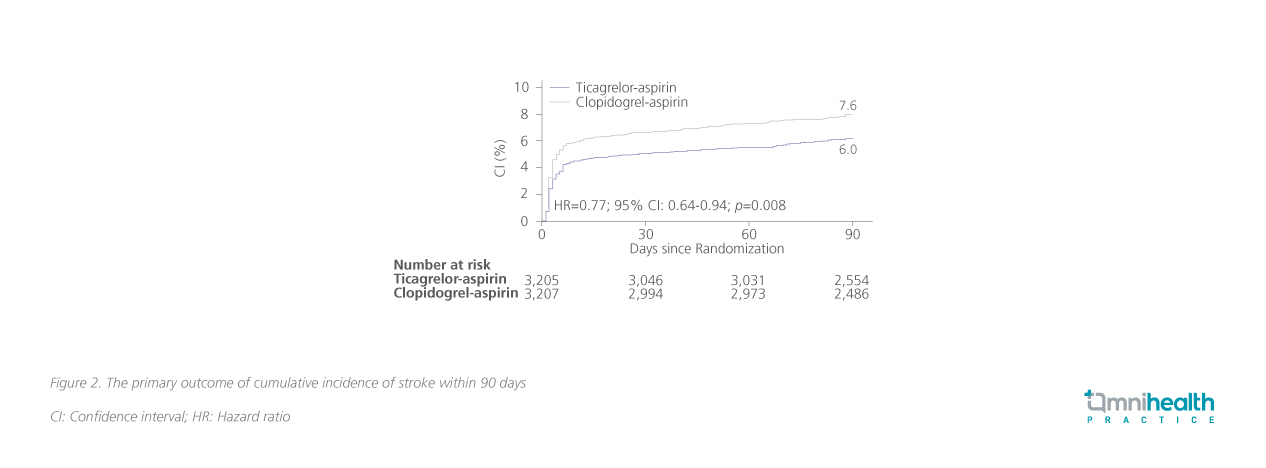
Moreover, aspirin-ticagrelor DAPT demonstrated comparable safety with aspirin-clopidogrel. The incidence of moderate or severe bleeding was 0.3% in both arms with no statistical significance being established (HR=0.82; 95% CI: 0.34-1.98; p=0.66).13 Incidence of adverse events (AE) (16.8 vs. 13.3%) and serious AEs (2.4 vs. 2.6%) were also somewhat uniform between the two treatment cohorts.13 The findings of the CHANCE-2 study established the foundations of ticagrelor being a potential alternative P2Y12 receptor antagonist for DAPT, particularly among certain East Asians where the majority are carriers of CYP2C19 LOF alleles.13
Ticagrelor extends protection beyond patients with CYP2C19 LOF phenotype
The efficacy of ticagrelor as a component in DAPT has been demonstrated in the THALES trial, where patients were not recruited based on CYP2C19 LOF allele status.16 The THALES trial is a randomized, double-blinded trial in patients with a mild-to-moderate [National Institutes of Health Stroke Scale (NIHSS) score ≤5] acute non-cardioembolic IS, or TIA.16 A total of 11,016 participants were enrolled and randomized to receive 180mg ticagrelor loading followed by 90mg BD through day 30 (n=5,523) or a matching placebo (n=5,493) within 24 hours of symptom onset.16 Within 30 days after treatment initiation, the aspirin-ticagrelor arm had a significantly lower incidence of stroke or death than the aspirin-only arm (5.5 vs. 6.6%), indicating an overall 17% risk reduction (HR=0.83; 95% CI:0.71-0.96; p=0.02) (figure 3).16 Nevertheless, it should be noted that aspirin-ticagrelor treatment was associated with an increased risk of severe hemorrhage over aspirin alone (HR=3.99; 95% CI: 1.74-9.14; p=0.001).16
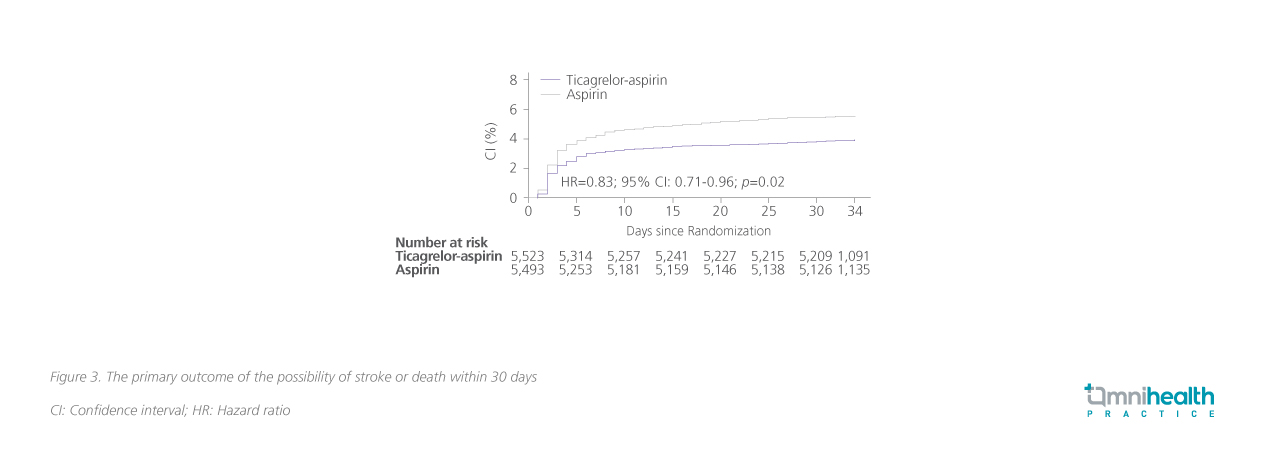
Striking the balance between bleeding and recurrent stroke risk
Ticagrelor exhibited comparable tolerability in both the CHANCE-2 and THALES studies. In the THALES trial, despite the increased risk of bleeding, risk-benefit analysis has shown that the number needed to treat to prevent one case of stroke or death is 92 while the number needed to harm that causes severe bleeding is much higher at 263.16 When the comparator arm is also a DAPT regimen instead of monotherapy, as it is in CHANCE-2, the risk of moderate or severe bleeding has been further attenuated to non-statistical significance.
Despite the promising potential of ticagrelor as a replacement for clopidogrel, clinicians should remain alert to the elevated risk of bleeding.13,16 Dr. Tang reiterated that patients often have complex medical histories and CYP2C19 status is just one point of consideration. Balancing the risk of bleeding and stroke risk is “as much an art as it is science”, having to evaluate each patient on a case-by-case basis.
Conclusion
In conclusion, CYP2C19 LOF allele carriers are poor or intermediate metabolizers of clopidogrel, which constitute a fair proportion (~60%) of the Hong Kong population.4,14 Ticagrelor has demonstrated a promising efficacy both in lowering the risk of stroke and death among patients regardless of their CYP2C19 phenotype and is proven to be effective in Chinese populations with CYP2C19 LOF.13,16 Dr. Lau reckoned that although ticagrelor may theoretically be more suitable for Asian patients, clinicians should cautiously consider the risk-benefit ratio before administering it as a DAPT.

