CONFERENCE UPDATE: ESMO 2023
Pembrolizumab + CCRT offers survival benefits to patients with previously untreated locally advanced cervical cancer: The ENGOT-cx11/GOG-3047/KEYNOTE-A18 study
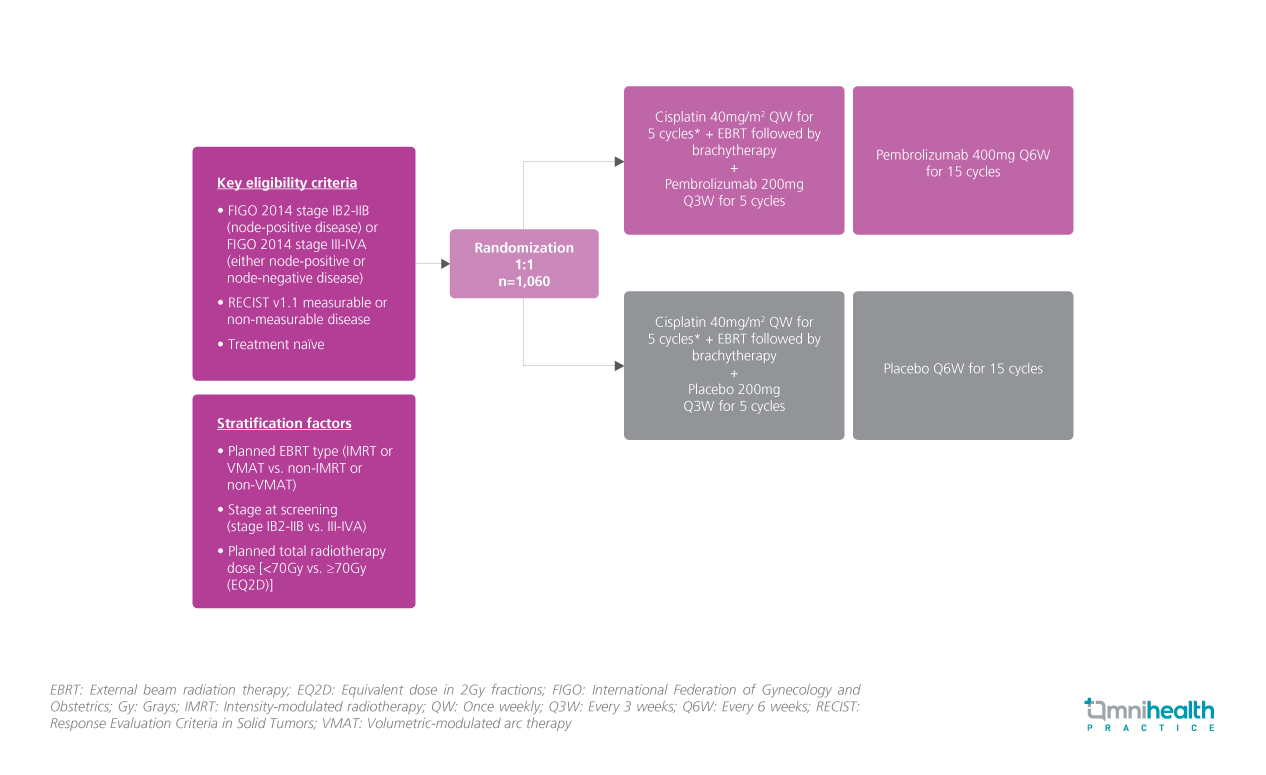
STUDY DESIGN
Previous clinical trials have shown that pembrolizumab, a programmed cell death-1 (PD-1) inhibitor, offered clinical benefits and a manageable safety profile to patients with recurrent or metastatic cervical cancer, either as a monotherapy or in combination with platinum-based chemotherapy CT.1 In the ENGOT-cx11/GOG-3047/KEYNOTE-A18 study, the efficacy and safety of pembrolizumab combined with concurrent chemoradiotherapy (CCRT) for patients with previously untreated, high-risk, locally advanced cervical cancer were assessed.1
In this randomized, double-blinded, multicentre phase 3 study, 1,060 patients with either stage IB2-IIB (node-positive) or stage III-IVA (either node-positive or negative) cervical cancer were enrolled across 176 sites in 30 countries.1 Patients were randomized to receive a CCRT regimen [cisplatin 40mg/m2 QW + external beam radiation therapy (EBRT) followed by brachytherapy] with either pembrolizumab 200mg (n=529) or placebo every 3 weeks (n=530) for 5 cycles. In addition, 15 cycles of pembrolizumab 400mg or placebo Q6W was offered as the maintenance therapy for their respective treatment groups.1
At a median follow-up of 17.9 months (range: 0.9-31.0), the primary endpoints progression-free survival (PFS) (per RECIST v1.1) by investigators or histopathologic confirmation and overall survival (OS) were measured.1 Secondary endpoints which included 24-month PFS, overall response rate (ORR), patient-reported outcomes (PROs), and safety, were also measured.1
FINDINGS
| Primary endpoints: |
|
|
|
| Secondary endpoints: |
|
|
|
|
|
| Safety: |
|
|
|
|
"These data support pembrolizumab + CCRT as a new potential standard of care for patients with newly diagnosed, previously untreated, high-risk, locally advanced cervical cancer"
Dr. Domenica Lorusso
Fondazione Policlinico Universitario Agostino Gemelli IRCCS/Catholic
University of Sacred Heart,
Rome, Italy

