CONFERENCE UPDATE: ESC 2023
Sustained clinical remission rates with 2-years of benralizumab treatment among SEA patients in XALOC-1 trial
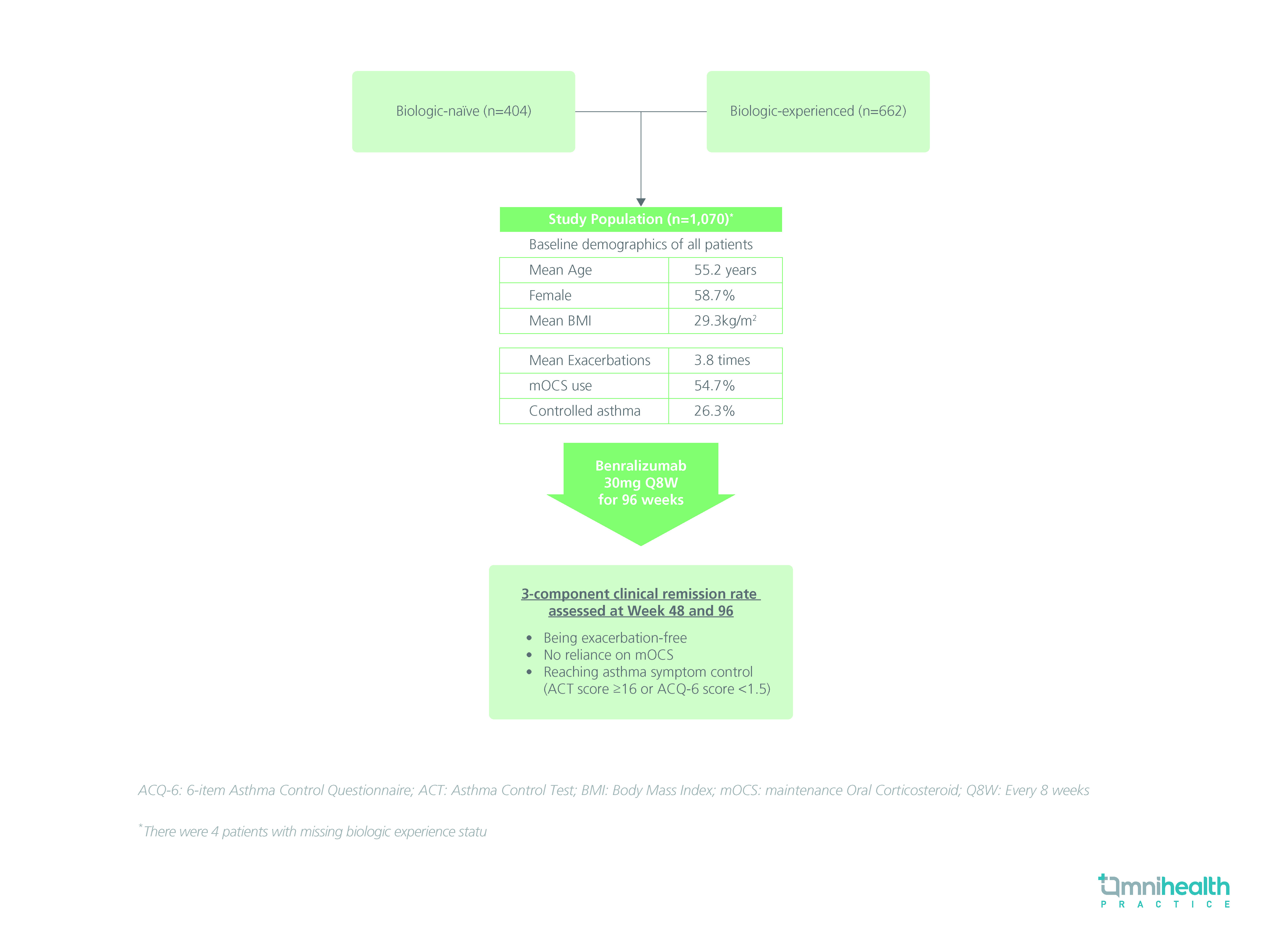
STUDY DESIGN
Despite high-dose inhalation therapies, disease control of severe eosinophilic asthma (SEA) has always been suboptimal, leading to frequent exacerbations and swift deterioration in lung function, requiring supplementation with high dose oral corticosteroids (OCS).1 With the emergence of biologic therapies such as benralizumab, clinical remission has become a realistic goal for patients with SEA.1 To understand the long-term effects of biologics treatment for SEA, the XALOC-1 study was conducted to analyze the 2-year efficacy of benralizumab in patients with SEA, regardless of prior experience with biologics.1
The XALOC-1 study was a retrospective, multinational program that recruited 1,070 adult patients with SEA, of which 61.9% of patients reported having no prior biologics treatment.1
During the 12-month baseline study period, the mean frequency of exacerbations in the study population was 3.8 times, with 54.7% of patients dependent on maintenance OCS (mOCS) and only 26.3% of patients retaining control over asthma symptoms [defined as having an Asthma Control Test (ACT) score ≥16 or 6-item Asthma Control Questionnaire (ACQ-6) score <1.5].1
The study population was then given benralizumab 30mg for every 8 weeks (Q8W) and was followed-up for 96 weeks.1 The primary clinical outcome of this study was a 3-component clinical remission rate assessed at weeks 48 and 96.1 The 3 components included being exacerbation-free, no mOCS use, and maintaining symptomatic control of their asthma (ACT score ≥16 or ACQ-6 score <1.5).1
| Primary endpoint: |
|
|
|
|
|
|
|
"Clinical remission is a realistic, sustainable goal up to 2 years for patients with SEA receiving benralizumab, and can be achieved regardless of prior biologic experience"
Dr. Girolamo Pelaia
Università Magna Graecia,
Catanzaro,
Italy

