CONFERENCE UPDATE: ERS 2023
Dupilumab demonstrates significant efficacy and safety outcomes for patients with COPD and type 2 inflammation: The phase 3 BOREAS trial
STUDY DESIGN
Patients with chronic obstructive pulmonary disease (COPD) and type 2 (T2) inflammation are often burdened with an elevated risk of exacerbation and decline in lung function, resulting in subsequent morbidity and death.1 Novel biologics like dupilumab, a human monoclonal antibody that blocks interleukin (IL)-4 and IL-13 receptors, display a promising potential in alleviating T2 inflammation.1 As such, the BOREAS study was conducted to investigate the efficacy and safety of dupilumab in patients with moderate or severe COPD with T2 inflammation.1
The BOREAS study was a randomized, double-blinded, placebo-controlled, phase 3 study that enrolled a total of 939 patients with moderate-to-severe COPD and a blood eosinophil count of ≥300cells/µL.1 The study population was randomized to receive either dupilumab 300mg (n=468) or placebo (n=471) subcutaneously (SC) once every 2 weeks (Q2W) for 52 weeks.1 The primary endpoint of this study was the annualized rate of moderate-to-severe exacerbations from baseline to week 52.1 Secondary endpoints included treatment outcomes in terms of patients’ lung functionality, quality of life, and safety, which included the change from baseline to weeks 12 and 52 in pre-bronchodilator (BD) forced expiratory volume in 1 second (FEV1) and scores of patient-reported questionnaires such as St. George’s Respiratory Questionnaire (SGRQ) as well as the Evaluating Respiratory Symptoms in COPD (E-RS: COPD) questionnaire.1
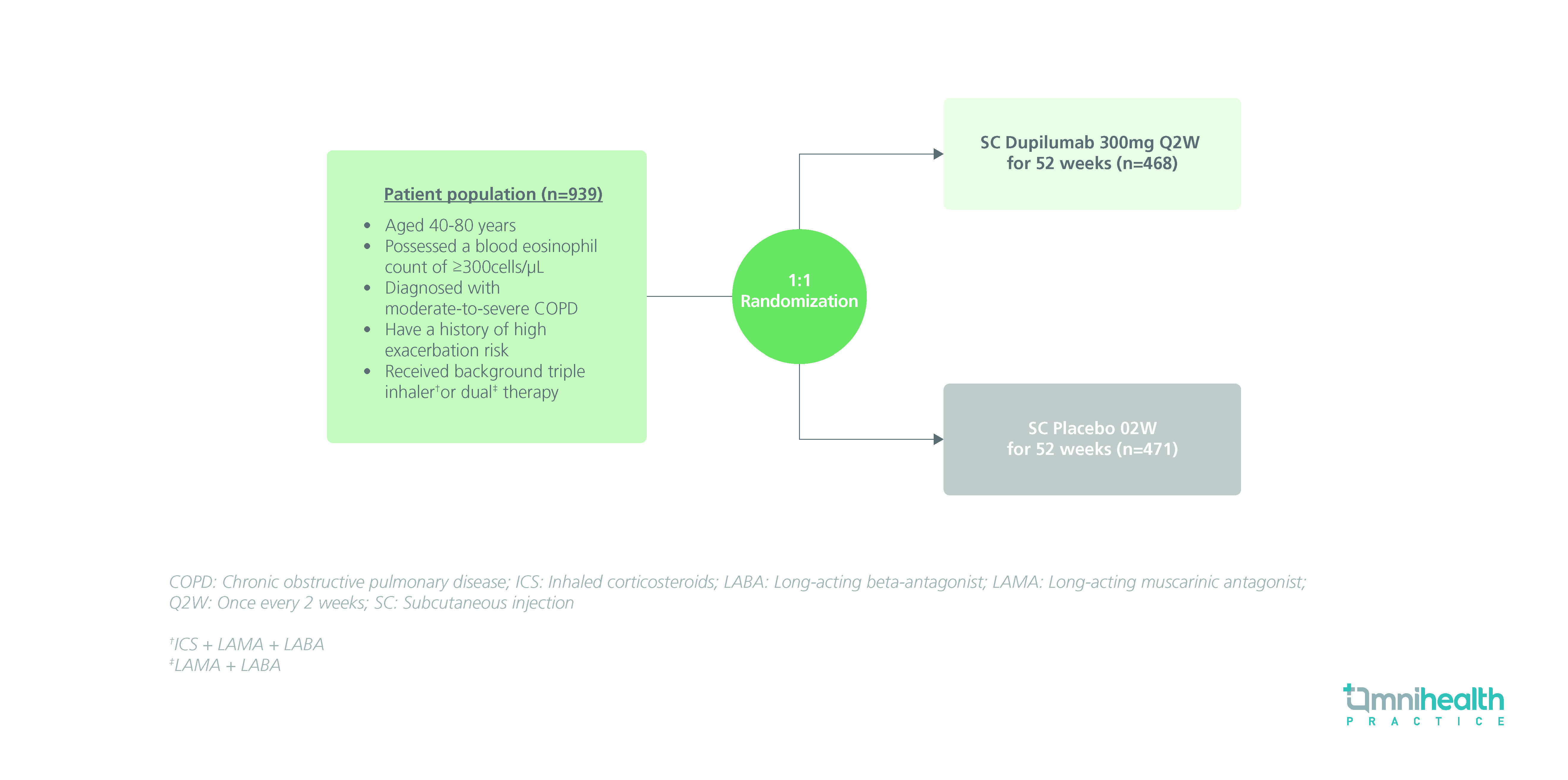
|
Primary endpoint: |
|
|
|
|
Secondary endpoints: |
|
|
|
|
Safety: |
|
|
|
|
"Dupilumab exhibited sustained and significant improvements in reducing exacerbations, maintaining lung function and quality of life in COPD patients with type 2 inflammation."
Professor Surya P. Bhatt
University of Alabama at Birmingham, Birmingham,
Alabama, United States

