CASE REVIEW
A case sharing: Exploring the long-term benefits of gilteritinib in FLT3+ RR AML patients
Patients with relapsed/refractory (RR) acute myeloid leukemia (AML) have a high risk of relapse and poor overall survival (OS) without hematopoietic stem cell transplantation (HSCT).1 The key to managing these patients is to induce remission so that HSCT becomes possible.1 This is particularly true for patients with FMS-like tyrosine kinase 3-positive (FLT3+) mutations who have relapsed on prior FLT3 inhibitors (FLT3i), as their prognosis is even more grim.2 In this setting, the addition of the second generation FLT3i like gilteritinib is crucial for patients with FLT3+ mutations to extend the OS regardless of the HSCT eligibility.2 In an interview with Omnihealth Practice, Dr. Singh, Gill Harinder Harry discussed a clinical case of a 50-year-old woman diagnosed with FLT3 internal tandem duplication (FLT3-ITD) AML who had relapsed on 2 lines of treatment. Rapid complete remission (CR) was induced with gilteritinib, leading to a successful HSCT. She then continued on gilteritinib maintenance and has remained relapse-free for 2 years after HSCT.
Background
Exploring the complexities of the RR AML landscape
Among AML patients, up to 40% of the younger ones eventually become primarily refractory to AML therapy, with that percentage increasing to 60% among elderly patients aged ≥60.1 Regardless of the age, RR AML patients who have failed induction chemotherapy have a dismal prognosis.2 However, a curative option is not completely out of the picture if they are fit enough to undergo HSCT.1 The current treatment goal for these patients is cytological remission, which is the prerequisite for the bridging procedure for HSCT.1
Emphasizing the significance of molecular testing at relapse and FLT3 mutations
Dr. Gill mentioned, “Molecular testing is very important at initial diagnosis, but it is even more important in identifying druggable targets at relapse.”Clonal evolution of AML is frequent, where the FLT3 mutation status is often different from the initial diagnosis.1 Molecular tests in the form of mutational screening and cytogenetic analysis should be performed again to check for new FLT3 mutations at the time of relapse.1 FLT3-ITD and tyrosine kinase domain (TKD) mutations are two of the most common mutations, at 25% and 10%, among AML patients respectively.1 These patients have poorer survival and a higher relapse rate of 40% despite receiving the first-line FLT3i therapy and they are both classified as intermediate risk according to the latest 2022 European LeukemiaNet (ELN) recommendations.3,4
Dr. Gill added that the addition of targeted therapy is becoming the new standard of care (SoC) in the treatment of RR AML patients, including those with FLT3 mutations. Gilteritinib is a second-generation, type I FLT3i which is associated with fewer off-target effects yet provides a wide coverage of both FLT3-ITD and FLT3-TKD mutations.5 The ADMIRAL study has shown high rates of complete remission with full or partial hematologic recovery (CR/CRh) with gilteritinib among patients with FLT3+ RR AML.2 Here Dr. Gill presented a case of an FLT3+ AML patient who had relapsed during consolidation therapy, with concurrent central nervous system (CNS) metastasis. The patient has been given a new chance for HSCT after achieving CR in the relapse with the use of gilteritinib.
Case sharing
A 50-year-old woman with no relevant past medical history was hospitalized for fever in 2019. On admission, she presented with leucocytosis and thrombocytopenia. She was suspected of having newly diagnosed AML, a diagnosis that was quickly confirmed after a bone marrow aspiration (BMA). Further investigations showed FLT3-ITD+ and normal cytogenetics with no chromosomal abnormalities, putting her in the ELN category of intermediate risk.
Given that a matched unrelated donor was identified, treatment was initiated immediately with the goal of inducing remission such that HSCT is possible. Induction therapy consisted of the 7+3 regimen (i.e., 7 days of standard-dose cytarabine + 3 days of daunorubicin) and consolidation was done with high-dose cytarabine on days 8-21. However, she experienced a relapse following the second cycle of consolidation. It was a severe relapse with evidence of leptomeningeal disease. Her spinal cord was also compressed leading to gait disturbances and she was no longer ambulatory. BMA showed 40% leukemic blasts with persistence of FLT3-ITD on molecular studies. Noting that the second-line chemotherapy [i.e., the FLAG regimen: Fludarabine, high-dose cytarabine, and granulocyte colony-stimulating factor (G-CSF)] did not induce remission by week 4 with persistence of FLT3-ITD, Dr. Gill initiated gilteritinib 120mg once daily (QD) as the third-line of therapy.
After only 30 days of gilteritinib treatment, the patient’s CNS disease disappeared and she has managed to attain CRh with a platelet count of 100x109/L, a neutrophil count of 1x109/L, and a hemoglobin count of 10g/dL. During this time, one episode of cytopenia lasting 6 weeks attributed to concurrent chemotherapy was reported,
requiring a dose reduction to 80mg QD. HSCT was conducted in December 2020, 2 months after CR, and a full complete blood count recovery was achieved (hemoglobin: 12g/dL; leucocyte count: 5.3 x 109/L; platelet count: 165 x 109/L). As the patient’s FLT3 status remained positive after HSCT, gilteritinib maintenance was continued to prevent further relapses. The dose of maintenance therapy was 80mg QD to accommodate concurrent immunosuppressive therapy of cyclosporin and was maintained past the 2-year post-transplant mark when the risk of relapse is highest. She is currently stable and is followed up once every 2 weeks (Q2M) with blood labs drawn every month.
Besides the one instance of cytopenia, the patient had reported minimal adverse effects (AEs), including minor muscle pain with no gastrointestinal (GI) upset or cardiac/hepatic/renal AEs. No additional AEs were reported over the 2 years of therapy in the post-HSCT setting.
Discussion
Relation between higher CR rate and eligibility for HSCTs
Dr. Gill highlighted that for patients who plan to undergo HSCT, like the patient in the current case, gilteritinib provides a longer-lasting CR/CRh which is an important consideration due to the time required for HSCT preparation.
In the ADMIRAL phase 3 trial, RR AML patients with FLT3 mutations were randomized 2:1 to receive gilteritinib 120mg QD (n=247) or salvage chemotherapy (n=124).2 The primary endpoints of ADMIRAL were OS and the percentage of patients achieving CR/CRh.2 A higher proportion of patients achieved CR and CR/CRh with gilteritinib vs. salvage chemotherapy respectively.2 The proportion of patients who achieved CR/CRh and CR alone were doubled with gilteritinib vs. salvage chemotherapy [54.3% (n=134) vs. 21.8% (n=27) (Risk difference=32.5%; 95% CI: 22.3-42.6) and 21.1% (n=52) vs. 10.5% (n=13) (Risk difference=10.6%; 95% CI: 2.8-18.4) respectively].2
In a follow-up (FU) done 37.1 months after the final analysis, the median duration of CR was 23 months [interquartile range (IQR): 4.9-not evaluable (NE)], with the median duration of CR/CRh at 4.6 months (IQR: 1.9-24.0).6 This has translated into a higher percentage of patients undergoing HSCT in the gilteritinib arm at 25.5% (n=63/247) vs. 15.3% (n=19/124) in the salvage chemotherapy arm.2
Gilteritinib extends OS regardless of HSCT
At the FU analysis, median OS was significantly longer with gilteritinib (9.3 months) vs. salvage chemotherapy (5.6 months) (HR=0.665; 95% CI: 0.518-0.853; ptwo-sided =0.0013) (figure 1).6
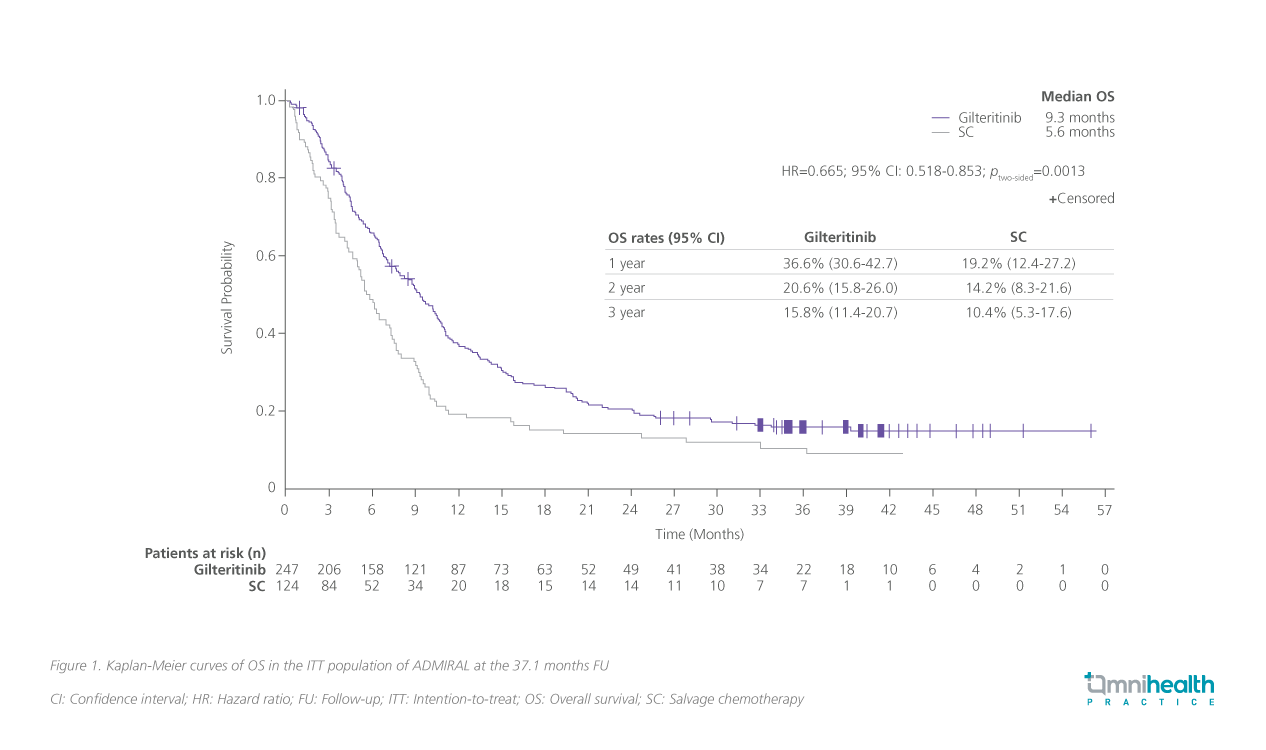
The OS benefit was consistent across subgroups stratified by age in the initial analysis where patients may be frail and not fit for HSCT.2 In the younger subgroup aged <65, there was a 39% risk reduction in death (HR=0.61; 95% CI: 0.43-0.86) while in the subgroup aged ≥65 years old, the risk reduction was maintained at 36% (HR=0.64; 95% CI: 0.44-0.95).2 Notably, when censored for HSCT, the OS benefit was not diminished at the time of transplantation (HR=0.58; 95% CI: 0.43-0.76).2
The improving safety profile of gilteritinib enables prolonged maintenance post-HSCT
Dr. Gill also reminded us that in the post-transplant setting, maintenance options, such as gilteritinib, should be considered. Notably, after 37.1 months FU of the ADMIRAL study, 62.5% (n=40) of patients who had undergone HSCT resumed gilteritinib after HSCT.6 Among patients who continued to receive post-HSCT gilteritinib maintenance, cumulative relapse rates at 37.1 months were 0% in patients who had achieved a CR response prior to HSCT (n=4) or CR/CRh prior to HSCT (n=9).6
Over the long term, gilteritinib has proven to be safe and had minimal AEs. “The most common AEs I’ve encountered are gastrointestinal AEs and are easily managed with antiemetics. Dermatologic or cardiac toxicities are rare.” Dr. Gill said. Among AEs of interest, increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were the most frequent.6 Other AEs of interest included myalgia, blood bilirubin increase, muscular weakness, prolonged QT interval, blood creatine phosphokinase increase, syncope, Hypoxia, pulmonary edema, pericardial effusion, gamma-glutamyl transferase increase, hyperbilirubinemia, and transaminase increased.6
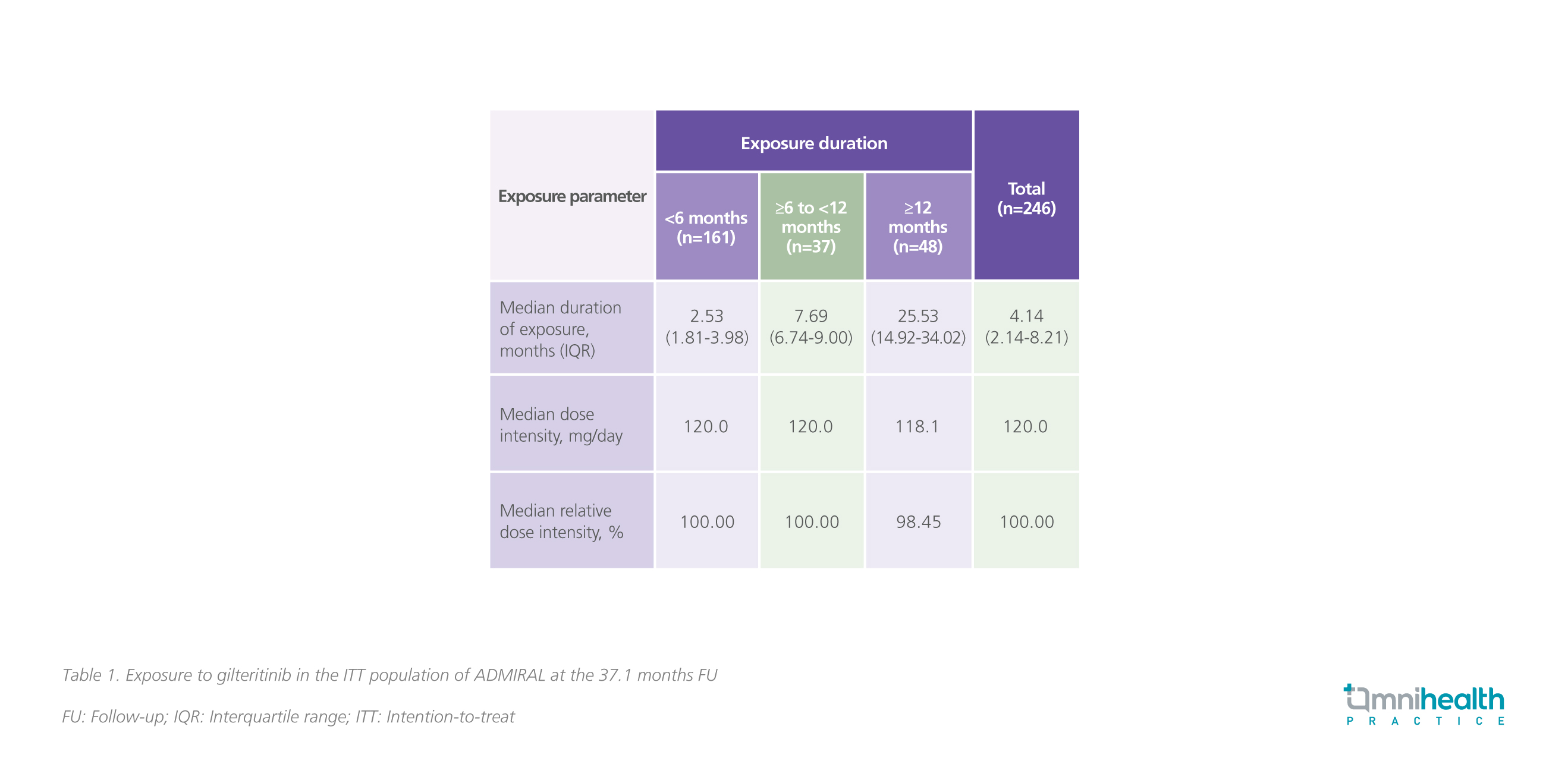
Overall, 5.3% of patients discontinued gilteritinib in the FU study.6 In the first year, 14.2% of patients experienced AEs of interest leading to dose reduction, with the number dropping to 8% in the second year.6 Rates of all AEs of interest continued to fall after 1 year of gilteritinib treatment with no new safety signals reported.6 This reassuring safety profile has extended the duration of use in the FU analysis, where 19.5% (n=48) had ≥12 months of exposure and 10.2% (n=25) had ≥24 months of exposure.6 Median dose intensity was kept at the full dose of 120mg/day in patients who were treated for less than a year and the median dose intensity remained similarly high at 118.1mg/day (98.45%) for those that were treated over a year (table 1).6
Conclusion
In conclusion, Dr. Gill reminded that it is important to perform molecular testing both at diagnosis and relapse to look out for druggable targets, especially FLT3 mutations. The addition of the new generation FLT3i like gilteritinib is crucial for patients with FLT3+ mutations who are eligible for HSCT, helping them achieve CR and prepare for HSCT, as demonstrated in the ADMIRAL study.6 Gilteritinib also extends the OS of RR AML patients regardless of the HSCT status, and offers a reassuring safety profile, allowing it to be resumed after HSCT, to prevent post-transplant relapse in FLT3-mutated AML.6 Hence, Dr. Gill recommended the use of gilteritinib at any stage of relapse, especially from first relapse onwards in patients with FLT3-ITD mutations.

