MEETING HIGHLIGHT
Transforming MCL care: The potential role of BTKi-based regimens in front-line treatment
At a recent symposium hosted by the Hong Kong Society of Haematology (HKSH) that discussed the current and future trends of the management of mantle cell lymphoma (MCL), Professor Simon Rule presented topics related to the trend of MCL management, the prognostic risk factors, and the risk stratification. He also reviewed the current treatment paradigm in MCL, highlighting the patient population with a high unmet need. Besides, he also discussed the different generations of Bruton tyrosine kinase inhibitors (BTKis) that have been introduced in the past decade, the data on BTKis in relapsed refractory (RR) MCL, the potential role of the BTKis and chemoimmunotherapy combinations, as well as the chemotherapy-free regimens for treatment-naïve MCL patients. At the symposium, Dr. Liu, Sung-Yu Herman, a local hematologist, shared the case of a male patient with indolent RR MCL in his sixties who remained in remission after completing 18 months of acalabrutinib treatment.
Utilizing practical prognostic factors to identify MCL patients with higher risk of relapse
MCL is considered one of the most aggressive and incurable lymphoid neoplasms.1 As the disease progresses, there will be an increasing number of genetic aberrations that affect alteration in the cell cycle regulation, the deoxyribonucleic acid (DNA) damage response mechanisms, and the activation of cell survival pathways, making it more difficult to treat relapsed patients.1
Several clinical prognostic factors are associated with the overall survival (OS) in MCL, such as age, the Eastern Cooperative Oncology Group performance status (ECOG PS), lactate dehydrogenase (LDH), and leukocyte count.2 The MCL International Prognostic Index (MIPI) classifies MCL patients into 3 prognostic groups based on OS, i.e., low-risk (median OS not reached), intermediate-risk (51 months), and high-risk (29 months).2 The degree of tumor cell proliferation has also been recognized as a strong biological prognostic factor in MCL.3 The combination of the Ki-67 index (>30%) and the MIPI led to the combined MIPI (MIPI-c), which showed a refined risk stratification into 4 groups, i.e., low (85%), low-intermediate (72%), intermediate-high (43%), and high (17%), integrating the most relevant prognostic factors available in clinical routine.3 Furthermore, mutations of histone-lysine N-methyltransferase 2D (KMT2D) and disruption of tumor protein 53 (TP53) genes are associated with an increased risk of progression and death.4 Hence, by adding these mutations to the MIPI-c backbone, a new prognostic index called the MIPI-genetic (MIPI-g) improved the model discrimination ability compared with the MIPI-c alone, defining the 3 risk groups.4 Prof. Rule pointed out that these indexes are very helpful in interpreting single-arm studies but not in clinical practice.
Progression of disease within 2 years of diagnosis (POD24) can also identify a patient population who has remarkably poor outcomes, irrespective of the prognostic information obtained at diagnosis or induction regimen administered.5 Yet, it is still not useful enough when it comes to clinical practice. The MCL35 assay, which measured 17 gene proliferation signatures, defines groups of patients with significantly different OS independent of the MIPI index, and that the results were reproducible.6 However, again, it is not very useful clinically.
There are 2 types of MCL, i.e., the classical or typical MCL with unmutated or minimally mutated immunoglobulin (IG) with sex determining region Y-box 11 (SOX11)-positive and the leukemic non-nodal MCL with hypermutated IG with SOX11-negative.7 The SOX11-negative MCL cases more often exhibit classic morphology with a lower Ki67 index than the SOX11-positive MCL cases.7 However, Prof. Rule stated that SOX11 is of no use in clinical practice since it cannot determine whether MCL is indolent.
The Nordic trial showed that MCL patients aged <66 years who were considered fit for autologous stem cell transplantation (ASCT) with TP53 mutation had a dismal outcome.8 For TP53-mutated patients, the median OS was 1.8 years, also showing a high relapse rate at 1 year (50%) compared with 12.7 years in the TP53-unmutated cases (p<0.0001).8 Hence, this subgroup of patients represents a huge unmet need.
BTKis as potential front-line treatment option for MCL
In fact, the treatment algorithm for MCL has not been changed for almost 8 years. “For frail elderly patients unfit for the first-line cyclophosphamide (CHOP) regimen, there is no real good alternative treatment, and there remains a high unmet need for MCL patients.9 This is really where the BTKis have a big potential advantage,” Prof. Rule stated.
BTKis were first developed for RR MCL nearly a decade ago, significantly improving the long-term clinical outcomes of patients. A pooled analysis of the RR MCL trial with an extended follow-up period of up to 7.5 years indicated that treatment with ibrutinib, an oral covalent BTKi, as second- vs. later-line treatment significantly extended the median progression-free survival (PFS) and increased the likelihood of complete response (CR).10 As the lines of treatment increase, the proportion of patients achieving a CR decreases.11 Prof. Rule stated that there is no reason not to administer BTKi after the first relapse, irrespective of what is happening.
Recently, there are 3 second-generation BTKi, of which acalabrutinib is one, being developed in RR MCL with similar efficacy but fewer side effects, primarily less cardiac toxicity, due to its greater selectivity to BTK, resulting in less off-target inhibition, according to Prof. Rule.12
BTKi combinations with chemoimmunotherapy for 37 treatment-naïve MCL
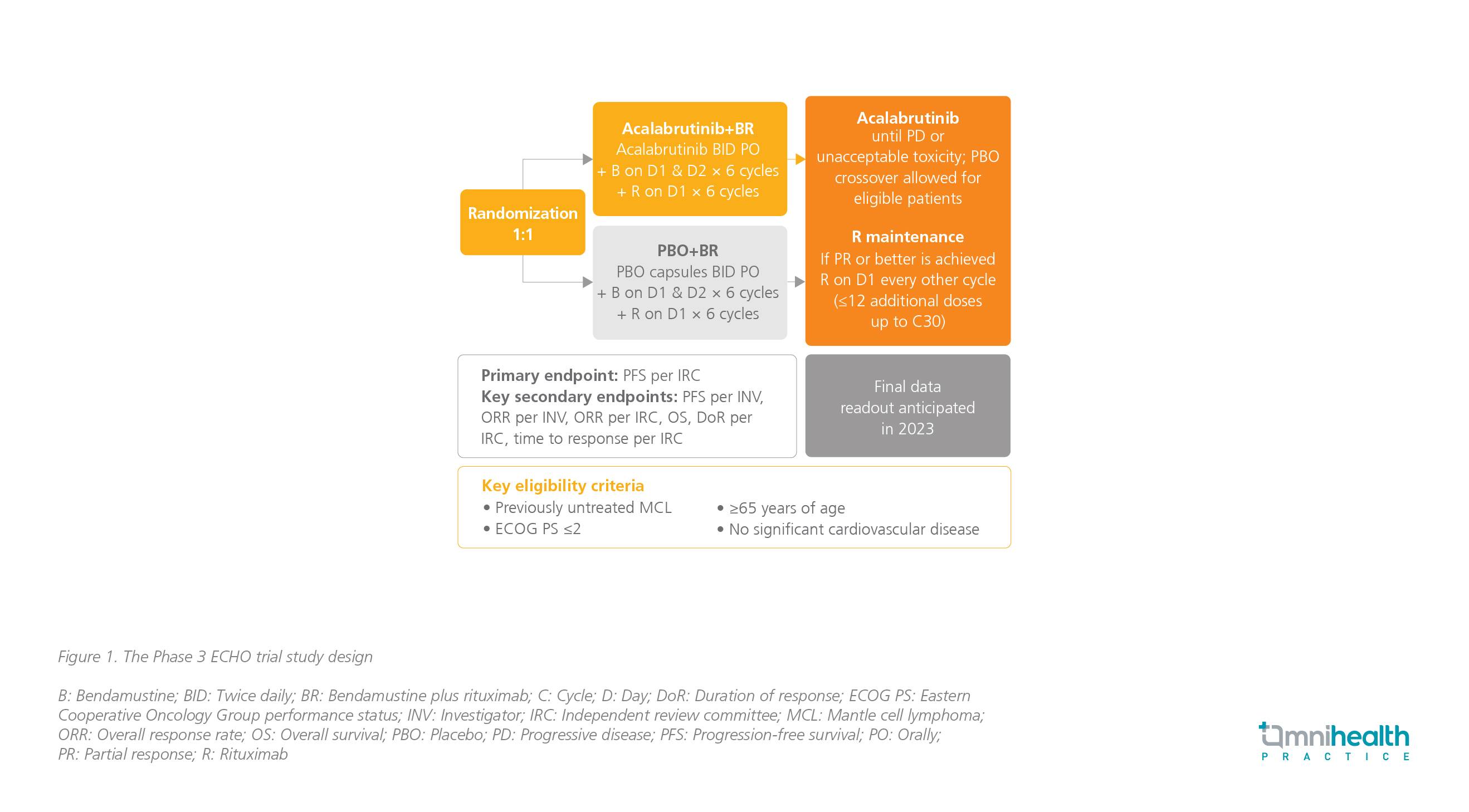
Besides, utilizing the combination of BTKis and chemoimmunotherapy for managing treatment-naïve MCL holds another great promise. The ECHO trial is an ongoing phase 3 trial that compares acalabrutinib plus bendamustine and rituximab (BR) with placebo plus BR in treatmentnaïve MCL patients (figure 1). The primary endpoint is PFS, and other key secondary endpoints are overall response rate (ORR), OS, duration of response (DoR), and time to response. Based on the previous experiences with acalabrutinib, Prof. Rule expected that patients in this study will remain on acalabrutinib longer when compared with ibrutinib, probably due to better safety, and he anticipated to have positive results from this study by next year.
Another international phase 3 study, the TRIANGLE study, was conducted to compare the safety and efficacy of adding BTKi to chemoimmunotherapy in patients with previously untreated MCL.13 The study found that adding BTKi in the maintenance phase of treatment may negate the need for ASCT, and this could potentially change the clinical practice.13
Case sharing by Dr . Liu
Dr. Liu then shared a clinical case to illustrate the effectiveness of acalabrutinib in managing relapses of the disease. In 2010, a 54-year-old male presented with enlarged supraclavicular (SCF) and axillary lymph nodes (LNs), measuring approximately 3.5cm in diameter. A biopsy of the left SCF LN confirmed MCL with a Ki-67 index of 30%. Positron emission tomography/ computerized tomography (PET/CT) showed multiple LNs above the diaphragm, with a maximum standardized uptake value (SUVmax) of up to 13. The patient had an MIPI of 6.1, indicating intermediate risk with a median OS of 58 months. He was treated with R-CHOP for 6 cycles and achieved CR. Radiotherapy (RT) was administered to the left SCF and left axilla, resulting in remission for 7 years.
In 2017, the patient noticed a new left parotid LN, measuring 8.8cm x 8.6cm x 11.6cm. PET/CT identified hypermetabolic lesions and bone marrow aspirate (BMA) showed results consistent with relapse of MCL. The patient received 6 cycles of BR every 4 weeks. The treatment was well tolerated, without significant adverse events (AEs) observed. PET/CT and BMA indicated metabolic remission. As the patient was ineligible for ASCT, R-maintenance was prescribed every 2 months for 12 doses. The patient remained in remission for another 3 years and 4 months.
In September 2021, the patient experienced significant weight loss, and a new right back mass measuring up to 3cm x 2cm was discovered. PET/CT revealed hypermetabolic lesions over the duodenum and intra-abdominal LN. A biopsy confirmed the second MCL relapse. Starting from October 2022, the patient received acalabrutinib 100mg twice daily. The back mass was completely resolved within 3 weeks. Leukocytosis and lymphocytosis were noted during the acalabrutinib treatment. Dr. Liu explained, “This is quite common with BTKi. When the LN shrinks, there is a spillage of lymphocytes into the circulation.” PET/CT after 6 months of acalabrutinib showed metabolic CR. In March 2023, the patient completed 18 months of acalabrutinib and responded well to the prescribed treatment. The patient has remained in remission as of June 2023.
Dr. Liu emphasized the necessity to prepare for the next relapse. The choice of the next line of therapy should consider factors such as the available treatments, comorbidities, age, functional status, etc.
Novel chemotherapy-free therapies for MCL
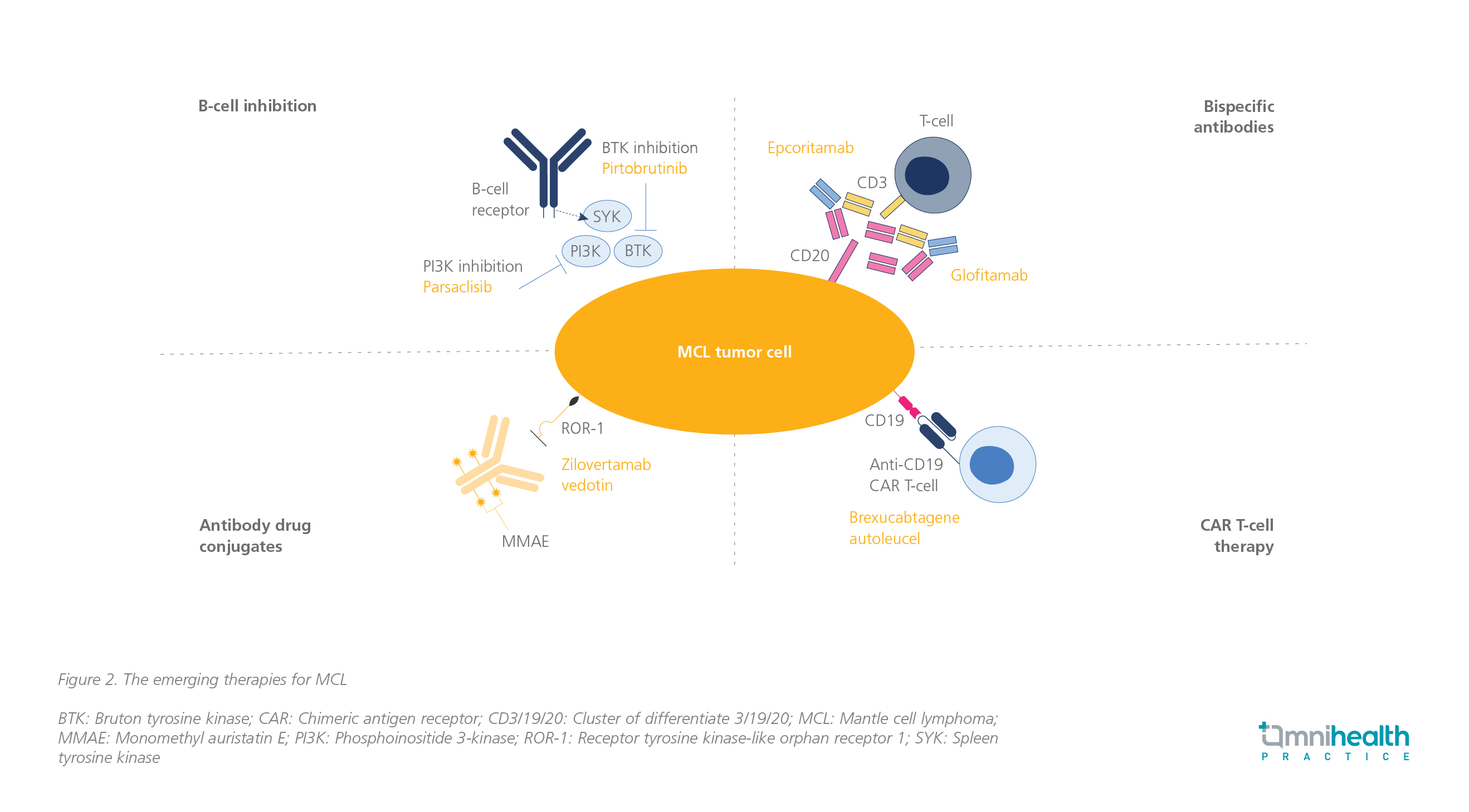
As an alternative to chemotherapy, researchers are now exploring emerging therapies for treating MCL including B-cell inhibition, bispecific antibodies, antibody-drug conjugates, and chimeric antigen receptor (CAR) T-cell therapy (figure 2).14 According to Prof. Rule, ongoing clinical trials (e.g., the ENRICH study, the MANGROVE study) are underway to investigate the potential of BTKi and B-cell lymphoma 2 protein (BCL-2) inhibitor combinations as an effective treatment for MCL.
Real-world data from the United States (US) showed good long-term outcomes with brexucabtagene autoleucel (brexu-cel), a CAR T-cell therapy, in patients with RR MCL.15 Nonetheless, patients with high-risk MIPI, high Ki-67, TP53 aberration, complex karyotype, and blastoid/pleomorphic variant still performed badly and had a shorter PFS after brexu-cel infusion.15 There remain concerns about whether the good response achieved with CAR T-cells is durable.
Additionally, the glofitamab monotherapy, a bispecific antibody, was shown to induce high response rates in MCL patients, even in those who failed prior BTKis.16 “Glofitamab could be a potential option for MCL and a combination of this drug is going to be important. It is clear that there is a shift away from chemotherapy as the evidence arises, and BTKis will hopefully become more widely available,” Prof. Rule asserted.
Conclusion
BTKi monotherapy has demonstrated promising and durable responses in RR MCL, particularly when given as an early line of therapy.10 Acalabrutinib, one of the second-generation BTKis, has similar efficacy and improved safety due to its higher selectivity with less off-target kinase inhibition.12 Patients with TP53-mutated MCL represent a high-risk population with less favorable outcomes with BTKis than the overall MCL population.8 Prof. Rule foresaw that the optimal combination in MCL would involve a BTKi, BCL2 positive/negative anti-cluster of differentiate 20 (BCL2 +/- anti-CD20), but its duration remains unknown, anticipating that the future combinations are likely to involve bispecific antibodies.9

