CONFERENCE UPDATE: ASCO 2023
Dostarlimab + carboplatin/paclitaxel shows consistent survival benefits in patients with primary advanced/recurrent EC: The BICR outcomes of the RUBY trial
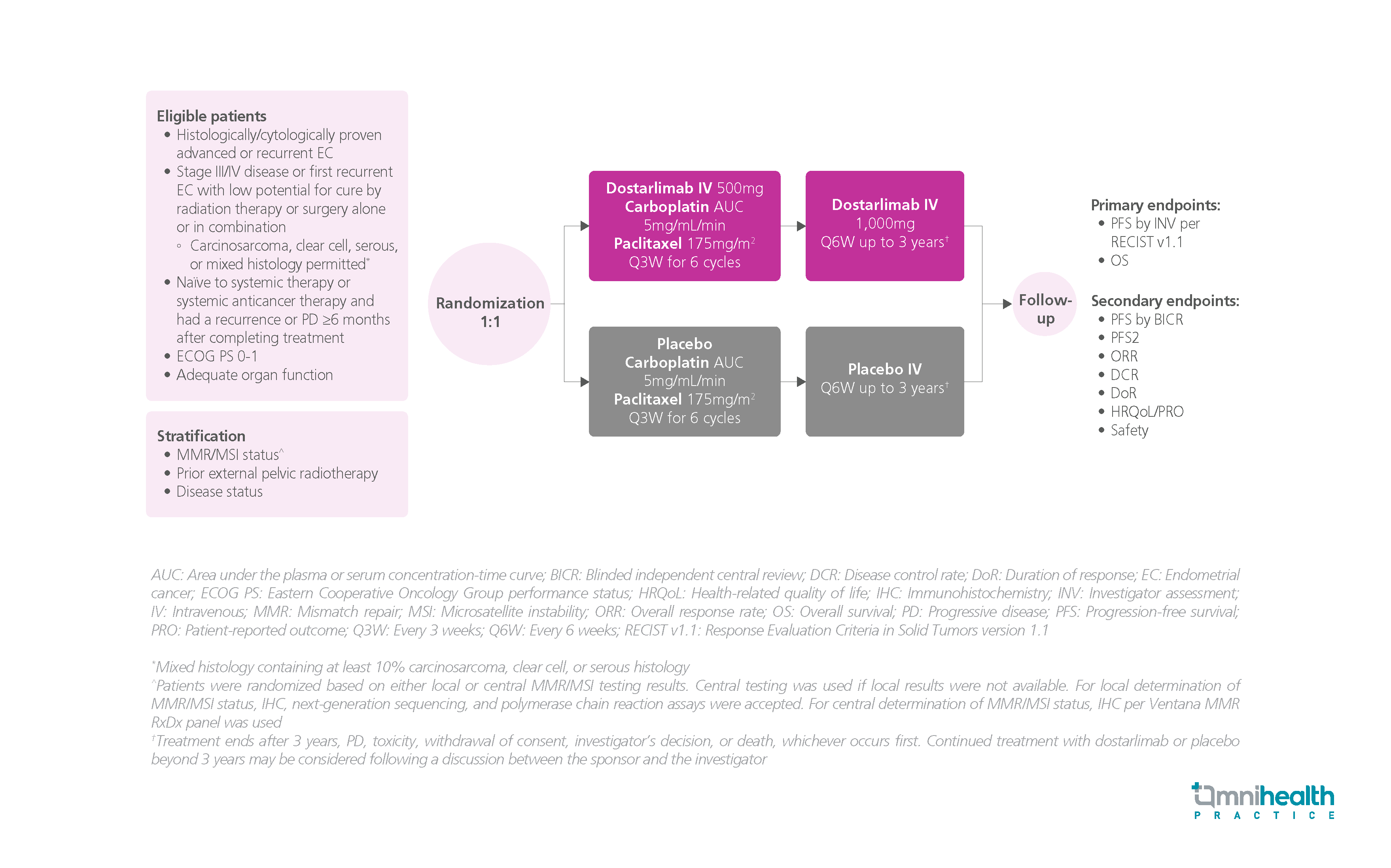
STUDY DESIGN
The use of dostarlimab monotherapy has shown to have a durable activity in treating mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) and MMR proficient/microsatellite stable (MMRp/MSS) previously treated endometrial cancer (EC).1 EC associated with dMMR/MSI-H is known as having high levels of tumor mutational burden (TMB) and tumor-infiltrating lymphocytes (TILs), making it more responsive to anti-programmed death-1 (PD-1) therapy monotherapy than MMRp/MSS.1 Additionally, chemotherapy combined with anti-PD-1 treatment has been demonstrated to improve immune system responses and clinical outcomes in various cancer types.1 Therefore, the combination of dostarlimab and chemotherapy could potentially enhance treatment outcomes in the dMMR/MSI-H and overall primary advanced and recurrent EC patient populations compared with chemotherapy alone.1
The RUBY trial was a phase 3, randomized, double-blind, multicenter study, in which a total of 494 patients with advanced or recurrent EC who had not previously received treatment or experienced a recurrence after receiving anticancer therapy were randomized 1:1 to receive either dostarlimab 500mg intravenously (IV) + chemotherapy of carboplatin-paclitaxel every 3 weeks (Q3W) for 6 cycles, followed by dostarlimab 1,000mg IV every 6 weeks (Q6W) for up to 3 years, or placebo + carboplatin-paclitaxel Q3W for 6 cycles followed by placebo Q6W for up to 3 years.1 The primary endpoint was progression-free survival (PFS) by investigator assessment (INV) and overall survival (OS).1 PFS by the blinded independent central review (BICR) was one of the secondary endpoints.1 The median follow-up was 24.79 months.1
The findings showed that patients receiving dostarlimab in combination with chemotherapy had clinically meaningful PFS benefit, assessed by BICR, with a 71% reduction in the risk of disease progression among the dMMR/MSI-H population and a 34% reduction in the overall population compared with patients receiving chemotherapy alone.1 Notably, PFS by BICR was consistent with that assessed by INV in both populations.1 Moreover, the objective response rate (ORR) by BICR and the duration of response (DoR) were both higher in the dostarlimab group than in the placebo group, and were consistent with the results evaluated by INV.1 Overall, the efficacy of dostarlimab with carboplatin-paclitaxel, along with its manageable safety profile, supported a favorable benefit-risk profile in patients with primary advanced or recurrent EC.1
FINDINGS
| Primary endpoints: |
|
|
|
| Secondary endpoints: |
|
|
|
|
| Safety: |
|
“Overall, the results supported a favorable benefit/risk profile of dostarlimab + chemotherapy in patients with primary advanced or recurrent EC”
Dr. Mathew A. Powell
National Cancer Institute-sponsored NRG Oncology,
Washington University School of Medicine,
St Louis, Missouri, United States

