CONFERENCE UPDATES: ASCO 2023
Cilta-cel in earlier lines improves outcomes over SoC in LEN-refractory MM patients
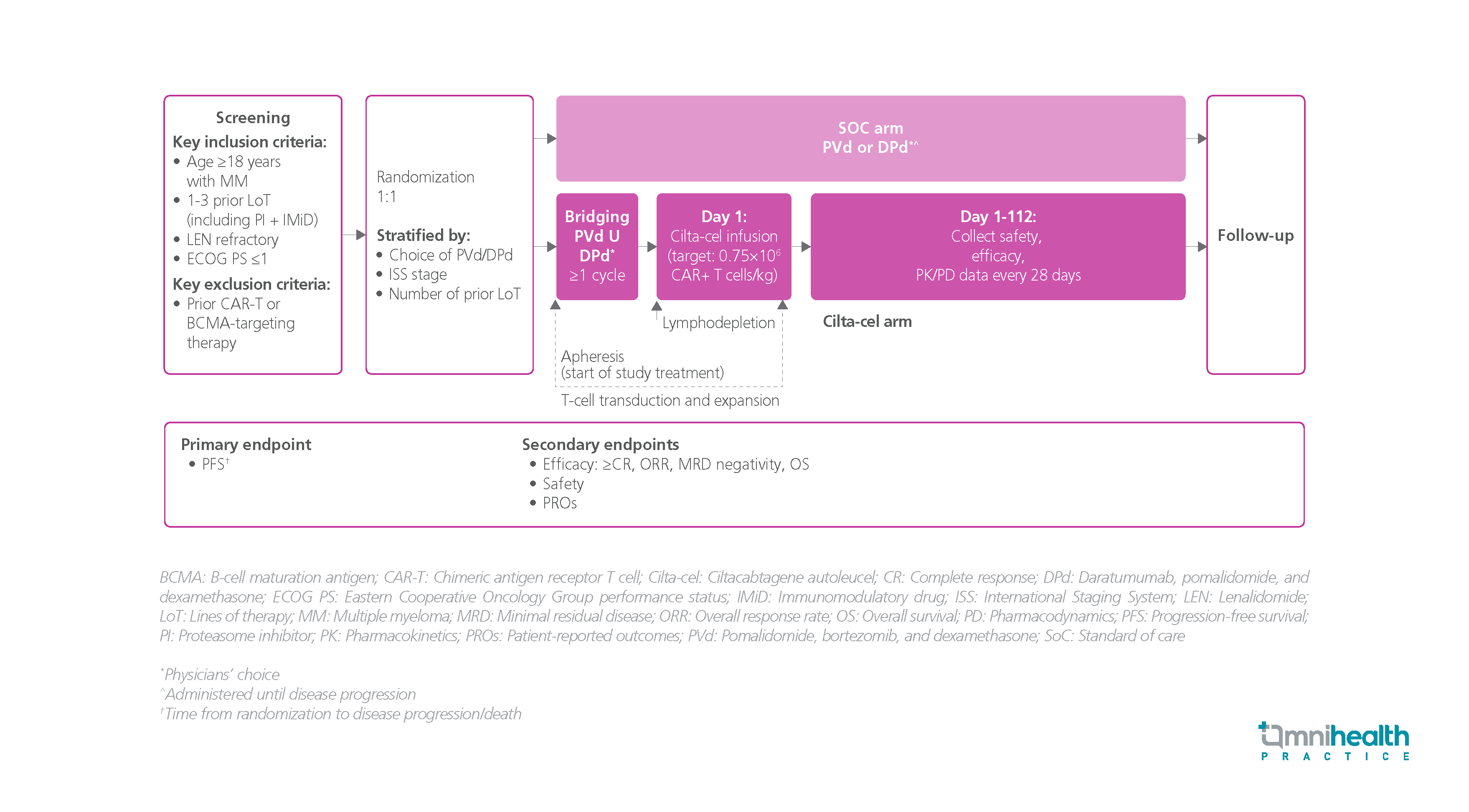
STUDY DESIGN
For patients with heavily pretreated lenalidomide (LEN)-refractory multiple myeloma (MM) who have received ≥3 lines of therapy (LoT), the efficacy of ciltacabtagene autoleucel (cilta-cel) has been demonstrated in previous studies.1 Cilta-cel is a dual-binding, B-cell maturation antigen (BCMA)-targeting chimeric antigen receptor T-cell (CAR-T) therapy, which has achieved a median progression-free survival (PFS) of around 3 years.1
A phase 3 CARTITUDE-4 study was conducted to evaluate the clinical benefits of cilta-cel in the LEN-refractory MM population with earlier LoT (i.e., 1-3 LoT).1 A total of 419 eligible patients were randomized 1:1 to receive either the cilta-cel arm (n=208) or the standard of care (SoC) therapy (n=211).1 The SoC was either the daratumumab + pomalidomide + dexamethasone (DPd) regimen or the pomalidomide + bortezomib + dexamethasone (PVd) regimen, while the cilta-cel arm received 1 cilta-cel infusion at a target of 0.75x106 CAR-T cells/kg.1 The primary efficacy endpoint was PFS.1 The secondary endpoints included overall response rate (ORR), complete response (CR) or more, minimal residual disease (MRD) negativity, overall survival (OS), and safety.1
The results demonstrated that cilta-cel achieved significant PFS benefits in the overall population with a 12-month PFS rate of 76% vs. 49% in the SoC arm and a relative risk reduction of 74% (HR=0.26; 95% CI: 0.18-0.38; p<0.0001).1 The PFS benefit was consistent across different subgroups.1 The cilta-cel arm also obtained a significantly higher ORR of 84.6% vs. 67.3% with SoC (OR=3.0; 95% CI: 1.8-5.0; p<0.0001).1 In addition, significantly more patients who received cilta-cel achieved the duration of response for 12 months (84.7% vs. 63.0%).1 Cilta-cel also improved the rates of overall MRD-negativity at 10-5 vs. SoC (60.6% vs. 15.6%; OR=8.7; p<0.0001).1
FINDINGS
|
Primary endpoints: |
|
|
|
| Secondary endpoints: |
|
|
|
|
|
|
Safety: |
|
|
|
|
“Cilta-cel has the potential to be a new SoC for patients with LEN-refractory MM after first relapse”
Dr. Binod Dhakal
Medical College of Wisconsin,
Milwaukee, Wisconsin, United States

