CONFERENCE UPDATE: ASCO 2023
Adjuvant ribociclib + ET shows survival benefits in HR+/HER2- EBC: The second interim analysis from the phase 3 NATALEE trial
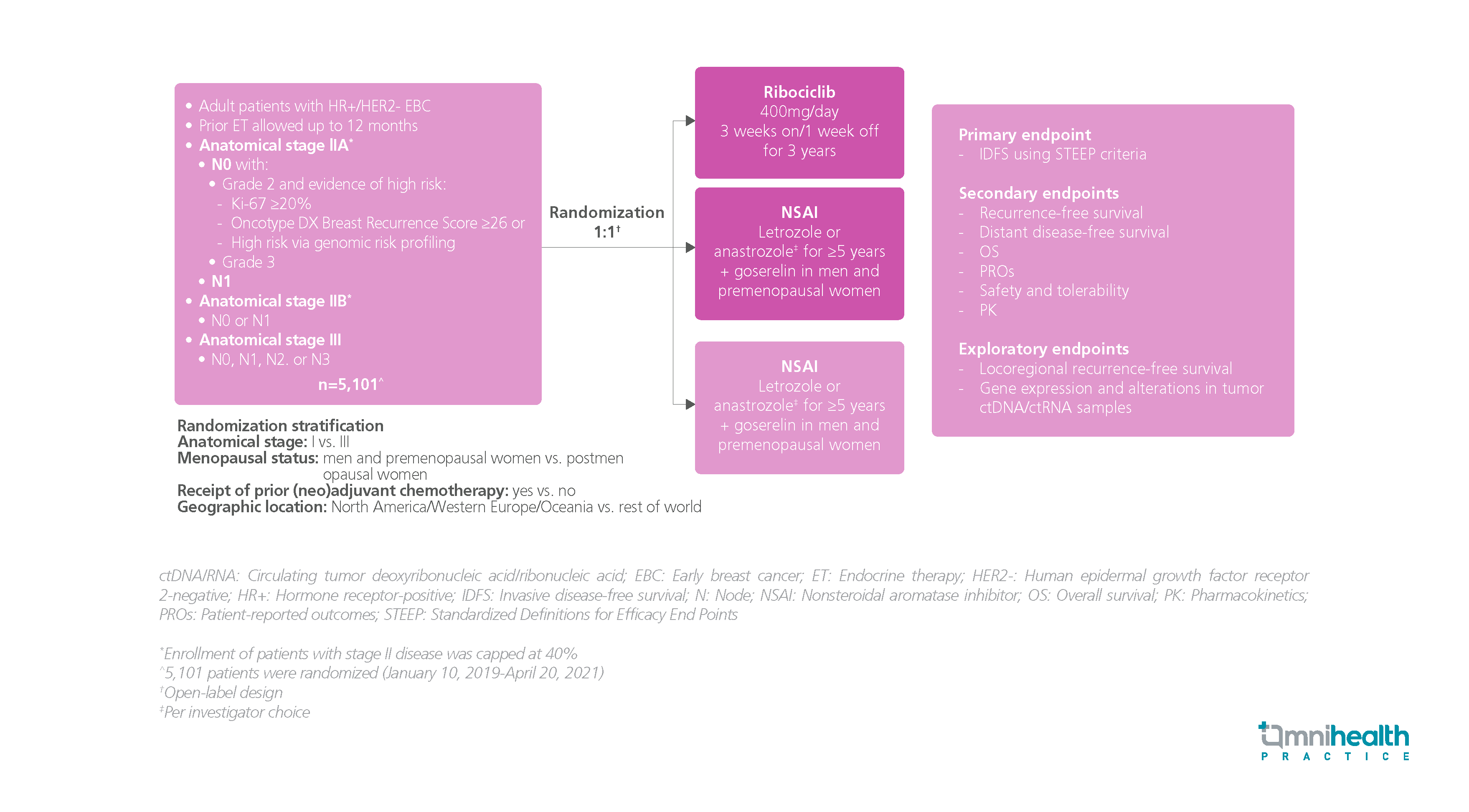
STUDY DESIGN
The goal of treating hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) early breast cancer (EBC) is curative.1 However, despite the use of the current standard of care (SoC) therapy, which consists of surgery with or without chemotherapy or radiation followed by 5-10 years of adjuvant endocrine therapy (ET), one-third of patients with stage II diseases and over half of patients with stage III diseases experience disease recurrence.1
The NATALEE trial was a randomized, controlled study designed to investigate the safety and efficacy of adjuvant ribociclib + ET in patients with HR+, HER2- stage IIA-III EBC, including those without nodal involvement.1 A total of 5,101 participants were recruited and randomized 1:1 to receive either ribociclib and nonsteroidal aromatase inhibitor (NSAI) (n=2,549) or NSAI alone (n=2,552).1 Ribociclib 400mg was given once daily (QD) on a “3 weeks on, 1 week off” schedule with a treatment duration of 3 years, while NSAI was given as the investigator’s choice of letrozole or anastrozole for ≥5 years with the addition of goserelin for men and premenopausal women.1 The primary endpoint was invasive disease-free survival (IDFS).1 The key secondary endpoints included recurrent-free survival, distant disease-free survival (DDFS), overall survival (OS), and safety, etc.1
The findings showed that adjuvant ribociclib + NSAI demonstrated a superior IDFS over NSAI alone with a relative risk reduction of 25.2% (HR=0.748; 95% CI: 0.618-0.906; p=0.0014).1 The IDFS benefits were consistent across different subgroups.1 Adjuvant ribociclib also showed a similar DDFS benefit vs. NSAI alone, reducing the risk of distant disease by 26.1% (HR=0.739; 95% CI: 0.603-0.905; p=0.0017).1 Ribociclib at a dose of 400mg was shown to be safe, well tolerated, and manageable.1
FINDINGS
|
Primary endpoint: |
|
|
|
|
Key secondary endpoints: |
|
|
|
|
Safety: |
|
|
“The NATALEE trial results supported ribociclib + NSAI as a new treatment of choice in a broad population of patients with stage ll or III HR+/HER2- EBC at the risk of recurrence, including patients with node-negative disease”
Dr. Slamon Dennis
David Geffen School of Medicine,
University of California,
Los Angeles, California, United States

