CONFERENCE UPDATE: ASCO 2023
Luspatercept demonstrates superiority to epoetin-α in ESA-naïve patients with transfusion-dependent low-risk MDS: Interim analysis of the COMMANDS trial
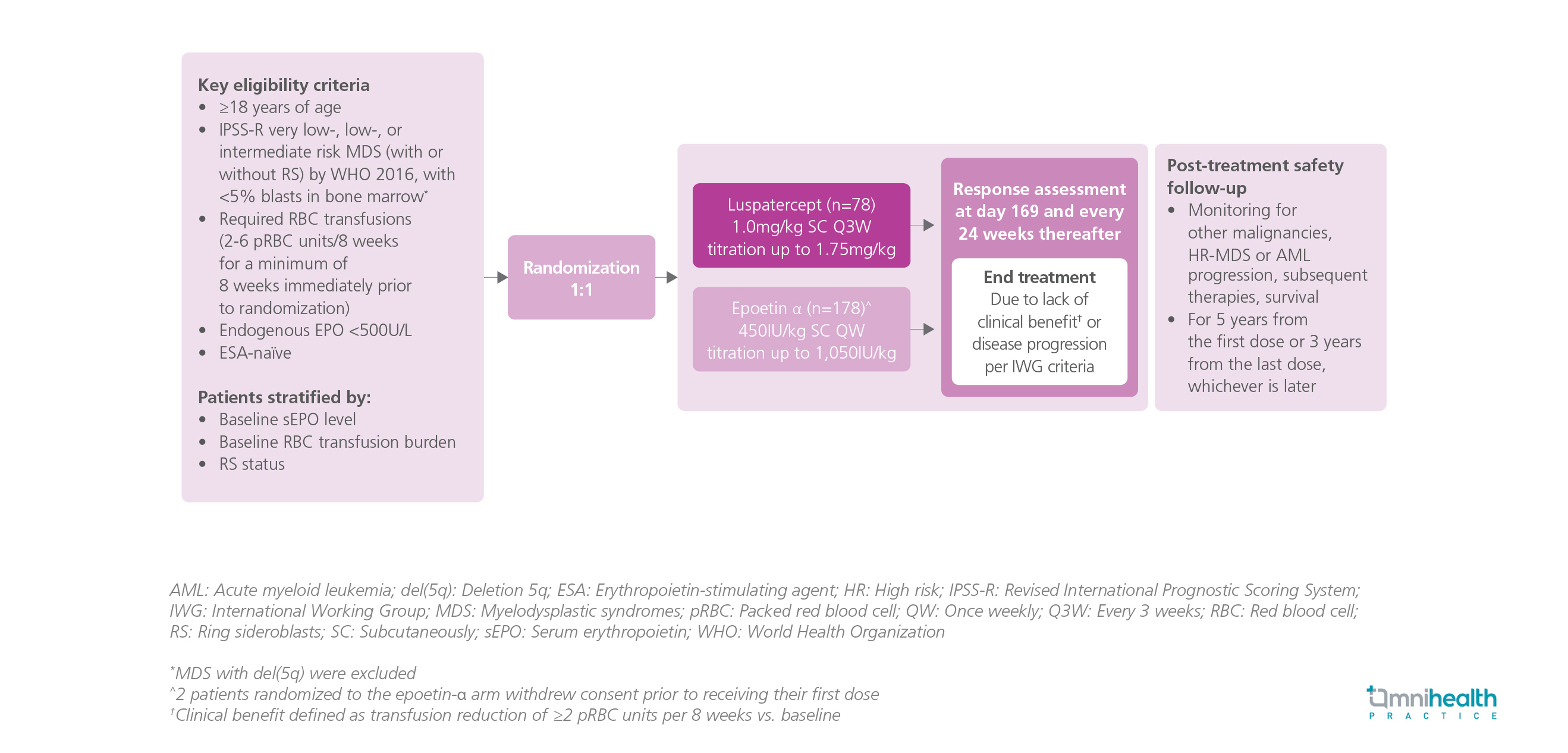
Study Design
Luspatercept has been previously proven to improve erythropoiesis in the MEDALIST trial and is beneficial to patients with low-risk myelodysplastic syndromes (MDS) with low transfusion burden, leading to an increase in mean platelet and neutrophil counts.1
The COMMANDS study was a global, phase 3, open-label, randomized trial designed to compare the efficacy and safety of luspatercept against epoetin-α for the treatment of anemia in erythropoiesis-stimulating agent (ESA)-naïve adult patients with transfusion-dependent low-risk MDS, as defined by the Revised International Prognostic Scoring System (IPSS-R).1 The patients were randomized 1:1 to receive luspatercept (n=178) 1mg/kg every 3 weeks (Q3W) or epoetin-α (n=176) 450IU/kg once weekly (QW).1 The composite primary endpoint was red blood cell (RBC) transfusion independence (TI) and concurrent mean hemoglobin (Hb) increase of ≥1.5g/dL.1 The secondary endpoints were hematologic improvement-erythroid (HI-E) response ≥8 weeks by the International Working Group (IWG) criteria, RBC-TI for ≥12 weeks, and RBC-TI at 24 weeks.1 The results were reported in the prespecified interim analysis of the first 24 weeks.
FINDINGS
|
Primary endpoint: |
|
|
|
Secondary endpoints: |
|
|
|
|
|
|
Safety: |
|
|
|
|
|
"Luspatercept is the first and only therapy that demonstrates superiority in a head-to-head study against ESAs, bringing a paradigm shift in the treatment of low-risk MDS associated anemia."
Dr. Guillermo Garcia-Manero
The University of Texas M.D. Anderson Cancer Center,
Houston, Texas, United States

