FEATURES
Beyond high-risk MM with Isa combination
Chromosome arm 1q gain (1q+) is among the most common cytogenetic abnormalities in multiple myeloma (MM).MM patients with 1q+ have recently been proven to have a worse prognosis than standard-risk patients, and 1q+ has also been recognized as an independent adverse factor for overall survival (OS) in MM patients.1 During the 2022 Annual Scientific Meeting of the Hong Kong Society of Myeloma, Professor Thomas G. Martin discussed the classification, current evidence, and clinical advancement of high-risk MM with reference to the relevant international guidelines or scoring system, highlighting the new inclusion of 1q+ in the most recent second revision of the International Staging System (R2-ISS). He also discussed a management algorithm for newly diagnosed as well as relapsed or refractory MM (RRMM) patients, and presented isatuximab (Isa) related data which showed a significant benefit when combining Isa with carfilzomib and dexamethasone (Kd), or pomalidomide and dexamethasone (Pd).
Defining high-risk MM
According to Prof. Martin, MM patients receive multiple lines of therapy until they eventually have refractory/relapse disease and die of MM. Unfortunately, this paradigm has not been changed yet, since a significant fraction of patients, especially those with high-risk diseases, are still not cured.
The International Myeloma Working Group (IMWG) InternationalStaging System (ISS) was the first risk score used to determine patient’s prognosis based on the beta 2 microglobulin (B2M)and albumin, and divided patients into 3 groups, namely low (I), intermediate (II), and high risk (III).2 A revised version of the ISS (R-ISS)added lactate dehydrogenase (LDH) and immunostaining fluorescence in situ hybridization (FISH) as additional features with a similar patient classification into the 3 groups.2
Mayo Clinic then developed its own risk classification system, the Mayo Stratification for Myeloma and Risk-AdaptedTherapy (mSMART), that defines high-risk MM as patients with high-risk cytogenetic abnormalities, including the t(4;14),t(14;16), t(14;20), deletion 17p (del 17p), p53 mutation, or1q+, R-ISS III, high plasma cell S-phase, and high-risk gene expression profiling (GEP).3,4 Prof. Martin reminded that ultra-high-risk patients were defined as having double-hit myeloma with 2 high-risk cytogenetic abnormalities, or triple-hit myeloma with ≥3 high-risk cytogenetic abnormalities and these patients have a very poor prognosis.4
Recently, a second revision to the international staging system (R2-ISS)has been developed by the European Myeloma Network (EMN), adding 1q+ as an ISS feature portraying a worse prognosis. The isolated risk features were assigned a risk score added to reach a score value, and classified patients into 4 groups, namely low (I), intermediate-low (II), intermediate-high (III), and high-risk (IV).5 High-risk patients with an R2-ISS ≥4 had a median OS of 34 months, which was significantly below today's standards, and a median progression-free survival(PFS) of only about 15 months.5
Similarly, a retrospective review examining 250 newly diagnosed patients in Hong Kong from 2006 to 2020 showed a 1q+ prevalence of 66.8%.6 Event-free survival (EFS) was shorter in patients with1q+ compared with patients without 1q+ (35 months vs. 55 months). Hence, 1q+ was recognized as an independent adverse factor for OS in the MM patients from Hong Kong.6
The management of newly diagnosed and RRMM patients
Before initiating treatment, newly diagnosed MM patients are required to be separated into transplant-eligible (i.e., fit) or nontransplant-eligible (i.e., frail). Prof. Martin suggested that the general rule of thumb is to give at least 8-12 cycles of induction treatment to frail patients or more cycles would be given if a patient continues to have debulking of their disease.
The goals of therapy for the transplant-ineligible can be variable but one main goal is the improvement of quality of life (QoL) and additional goals are aimed at getting a deeper remission and having potentially better OS among patients. Transplant candidates have more aggressive treatment goals. They usually receive 4-6 cycles of induction therapy followed by stem cell harvest and 1 or 2 stem cell transplantations (SCTs), with the goal of achieving the deepest remission, especially if they still have evidence of residual disease. The decision of administering post-SCT consolidation therapy depends on the presence of disease after the transplant. Almost all patients, no matter fit or frail, will proceed to maintenance therapy, to“maintain” their remission for as long as possible, especially if they have high-risk diseases.
In Prof. Martin’s opinion, the goal of transplant is to achieve minimal residual disease (MRD) negativity. The Intergroupe Francophone du Myelome (IFM) 2009 trial showed that patients who achieved MRD negativity, whether they had standard or high-risk disease, had better clinical outcomes than patients who remained MRD-positive. 7
When selecting therapy for relapse MM, Prof. Martin reminded the importance to consider patient-, treatment- and disease-related factors, i.e., convenience, patient comorbidities, and refractoriness to prior agents are often important considerations. Patients that relapse on maintenance therapy should be considered refractory to the maintenance regimen (even at low doses of maintenance).“It is about class switching between immunomodulatory drugs(IMiDs), proteasome inhibitors (PIs), and cluster of differentiation 38 (CD38) antibody therapy and selecting next-generation agents(i.e., Lenalidomide → pomalidomide). There are many different triplets to think about and choose from,” Prof. Martin said. There are no standard algorithms, but rather each patient needs to have therapy selected based on their preferences and their specific clinical situation. For instance, if a patient relapses during induction or soon after induction, a clinical trial testing a novel agent or combination may be the best option, but treatment with a triplet incorporating different agents (other than the ones they have been on) should be considered standard according to Prof. Martin. Patients undergoing SCT with a remission duration >2 years or ideally >5 years could consider a second autologous transplant. Overall, one should consider the timing of relapse, refractoriness or intolerance to agents, convenience and class switching.
Isa combination therapy improves survival outcomes in patients
Clinical trials have been the go-to therapy or important options for all eligible MM patients experiencing disease relapse and especially those with early relapse (relapsing during the induction or soon after induction <18 months). Several different novel agents and combinations are showing significant efficacy in clinical trials and one of the longest remission durations reported comes from the IKEMA study, utilizing the triplet Isa-Kd in RRMM.9 IKEMA was a prospective, randomized, phase 3 study, which included 302 RRMM patients with 1-3 prior lines of therapy.9 Patients were randomized 3:2 to the triplet of Isa + Kd (Isa-Kd) or the double of Kd.9 The primary endpoint of PFS was significantly higher with Isa-Kd (not reached) compared with a median of 19.15 months in the control group (HR=0.53; 99% CI: 0.32-0.89; pone-sided=0.0007).9 These data were recently updated after a median follow-up of 44months, showing an updated PFS with Isa-Kd of 35.7 months vs. 19.2 months in the Kd control group (HR=0.58; 95.4% CI: 0.42-0.79).10
With these updated data, Isa-Kd has demonstrated one of the longest PFS results with a PI-based backbone in the relapsed MM setting, with 42% reduction vs. Kd in the risk of progression or death (HR=0.58; 95.4% CI: 0.42-0.79).10 The subgroup analysis showed a consistent benefit across all subgroups including patients with high-risk cytogenetic status, Chromosome arm 1q21gain (1q21+), and refractoriness to lenalidomide (figure 1).9 With regard to all 1q+ subgroups, Isa-Kd also demonstrated significant improvement in median PFS and response rates.9"
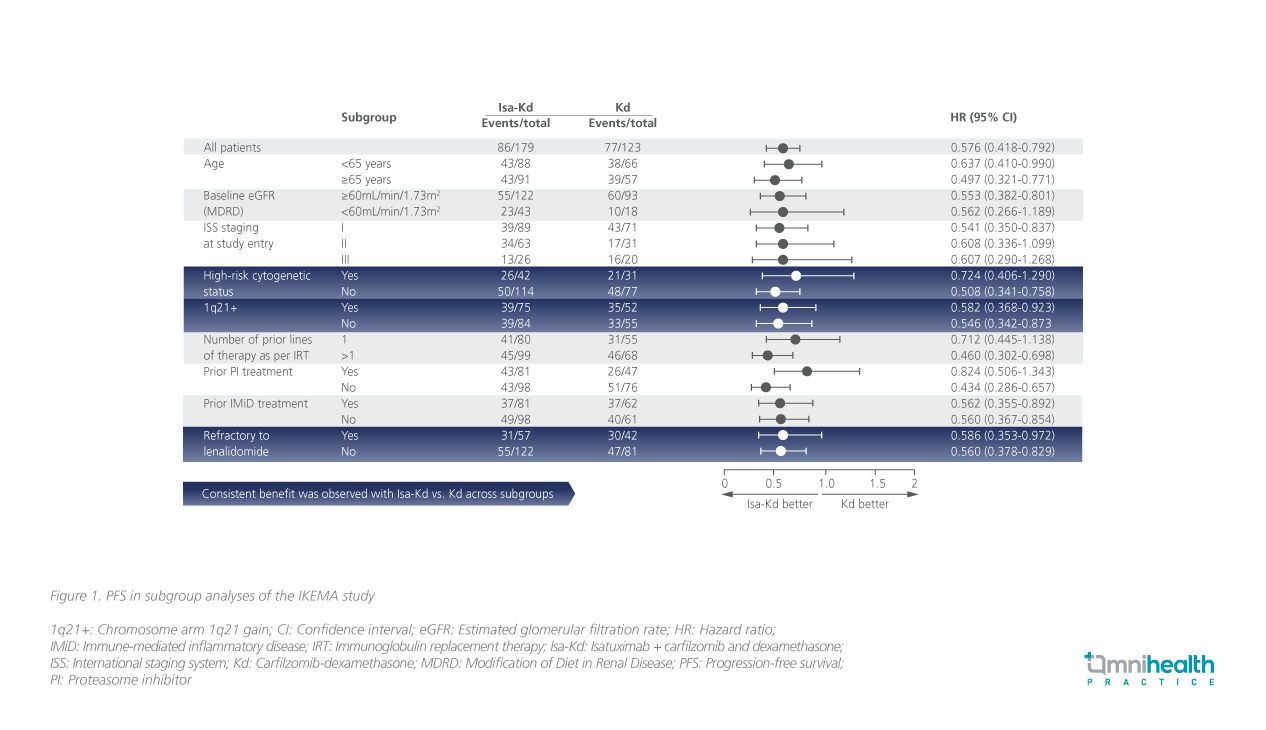
Isa-Kd is one of my favorite regimens for patients who have an early relapse, have not received CD38 antibody as part of frontline therapy, and/or have 1q21+ gain," Prof. Martin highlighted. The addition of Isato Kd also improved the depth and quality of response with higher rates of very good partial response (VGPR), complete response (CR), MRD negativity, and CR with MRD negativity.9
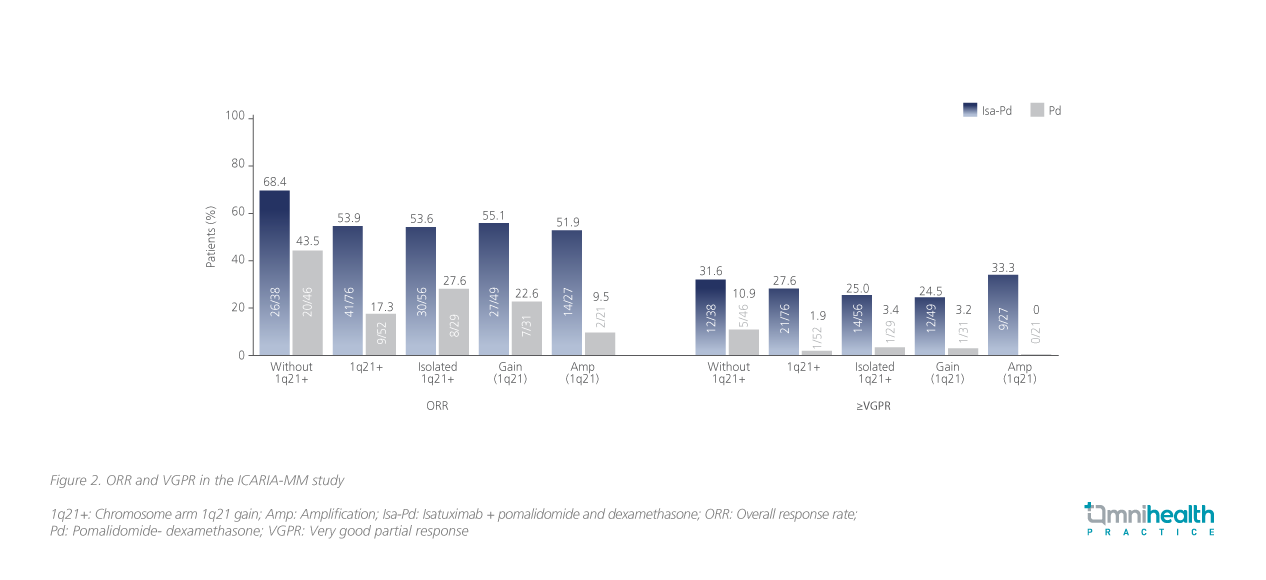
Apart from Isa-Kd, the ICARIA-MM study, a randomized phase 3 trial, examined the PFS benefit of the triplet of Isa + Pd (Isa-Pd) or Pdin RRMM patients who had received ≥2 previous lines of treatment, including lenalidomide and a PI, also showed benefit for the triplet therapy.11 After a median of 11.6 months follow-up, the primary endpoint of median PFS was 11.5 months in the Isa-Pd group vs. 6.5months in the Pd group (HR=0.596, 95% CI: 0.44-0.81; p=0.001).11The PFS benefit with Isa-Pd occurred in all prespecified subgroups, including patients with poor prognoses.11 Patients receiving Isa-Pdhad a higher OS of 72% than patients receiving Pd with an OS of63% (HR=0.687; 95% CI: 0.461-1.023; p=0.0631).11
In patients with 1q21+, Isa-Pd led to a median PFS of 9.5 months compared with a median PFS of 3.8 months with Pd (HR=0.40;95% CI: 0.25-0.63), and the median OS was also higher in theIsa-Pd group than in the Pd group (21.3 months vs. 13.9 months, HR=0.72; 95% CI: 0.48-1.07). All subgroups had consistently positive treatment effects from Isa-Pd with HRs ranging from0.5-0.6 (figure 2).11
Conclusion
The definition of high-risk disease continues to evolve and we now have the R2-ISS and mSMART assessments for NDMM, both of which have added 1q+ mutation as a new high-risk feature, and this is in addition to the previously defined features such as high-risk cytogenetics, high plasma cell S-phase, and high-risk GEP.3,4 The NDMM high-risk patients remain an unmet medical need and novel treatments are needed. Prof. Martin suggested that the goal of transplant-eligible NDMM patients should be to achieve and sustain MRD negativity as this is associated with improved outcomes in high-risk NDMM patients. Upon disease relapse, it is important to consider risk status as well as patient-, treatment-, and disease-related factors when selecting therapy for RRMM patients. Clinical trials are reasonable for all eligible MM patients, and recent trial results have shown that the addition of Isa to Kd or Pd led to significant improvements in PFS and OS, which were consistent in subgroups of patients, including those with high-risk diseases. CD38 antibody therapy will continue to play a vital role in the treatment of RRMM.9-11
MAT-HK-2300054-1.0-02/2023

