FEATURES
Real-world DATA confirm the effectiveness and tolerability of brivaracetam in patients with focal-onset seizures
Brivaracetam (BRV) has been approved in over 50 countries for focal-onset (i.e., partial-onset) seizures with or without secondary generalization.1 However, real-world evidence studies regarding the scope, location and number of BRV are limited, making it difficult to identify the trends of BRV response and tolerability across specific patient groups.1 At the American Epilepsy Society (AES) 76th Annual Meeting 2022, members from 3 teams presented their promising evidence on BRV in real-world settings.1-3
Prior anti-seizure medications (ASMs) ASM influence clinical response to newly administered BRV
To analyze the effect of the number of lifetime anti-seizure medications (ASMs) on the effectiveness and tolerability of BRV in patients with focal-onset seizures, EP0077 and EP0088 were undertaken as 12-month prospective studies in Europe and the United States (US), respectively, on patients taking BRV.2 Martin MS, et al. presented the post hoc analysis, which investigated the final data from EP0077 and EP0088 in patients aged ≥18 years with focal-onset seizures, no prior BRV treatment, ≥1 lifetime ASM, and ≥1 BRV dose during the study.2
The findings revealed that the main reason for BRV initiation was the lack of efficacy of current treatment in 73.3%, 78.8%, 78.2%, and 89.0% of patients with 1-2, 3-4, 5-6, and ≥7 lifetime ASMs, respectively.2 The median BRV modal dose was 100mg/day in patients with 1-2, 3-4, and 5-6 lifetime ASMs, and 150mg/day in patients with ≥7 lifetime ASMs.2 Over a 12-month period, the rate of BRV retention and seizure freedom decreased from 68.3% and 20.0% to 51.3% and 2.7% as the number of lifetime ASMs in patients increased from 1-2 to ≥7.2
According to the Kaplan-Meier estimates, patients with 1-2 and 3-4 lifetime ASMs were less likely to discontinue BRV or terminate the study than those with ≥5 lifetime ASMs.2 Patients with fewer lifetime ASMs were less likely to discontinue BRV due to the lack of efficacy (1.0% with 1-2 ASMs to 14.9% with ≥7 ASMs) or incidence of treatment-emergent adverse events (TEAEs) (5.0% with 1-2 ASMs to 15.2% with ≥7 ASMs).2 The incidence of TEAEs, drug-related TEAEs, behavioral adverse events (AEs), and BRV discontinuation due to TEAEs increased with the number of lifetime ASMs.2 AEs resulted in death in 1 (1.0%), 1 (0.6%), 1 (0.8%), and 1 (0.3%) patient of those with 1-2, 3-4, 5-6, and 7 lifetime ASMs, respectively.2
Additionally, patients with fewer lifetime ASMs before BRV initiation had numerically higher retention and seizure freedom rates and lower incidence of TEAEs and discontinuations due to either lack of efficacy or TEAEs.2 These results collectively indicated prior ASMs influence clinical response to newly administered BRV.
Patients with epilepsy receiving BRV monotherapy respond more effectively
In addition to the 2 prospective studies above, EXPERIENCE/EPD332 was a pooled analysis weighted towards highly drug-resistant cohorts.1 Data came from multiple independent non-interventional studies, involving patients with epilepsy who started BRV in routine clinical practice in Australia, Germany, Spain, and the US.1 Patient enrollment began when BRV became available in those countries, and patients had to begin BRV at a median dose of 100mg/day no earlier than January 2016 and no later than December 2019.1,2
The EXPERIENCE/EPD332 study assessed the effectiveness and tolerability of BRV in patients with epilepsy by the number of prior ASMs and BRV treatment type. Of 1,644 patients included in the treatment type study, at 3, 6 and 12 months, compared with polytherapy patients, monotherapy patients had numerically higher seizure freedom (58.1% vs. 21.2%, 34.5% vs. 17.5%, 36.0% vs. 14.5%, respectively, p-value not available) and continuous seizure freedom rates (58.1% vs. 21.2%, 31.0% vs. 15.3%, and 28.0% vs. 11.3%, respectively, p-value not available), indicating patients with epilepsy receiving BRV monotherapy responded more effectively, although clinically meaningful continuous seizure freedom was also observed in the polytherapy patients.1 There were no discernible numerical differences in ≥50% seizure reduction at 3, 6 and 12 months between monotherapy and polytherapy patients (35.7% vs. 32.1%, 26.7% vs. 36.9%, and 30.8% vs. 37.0%, respectively) (figure 1).1
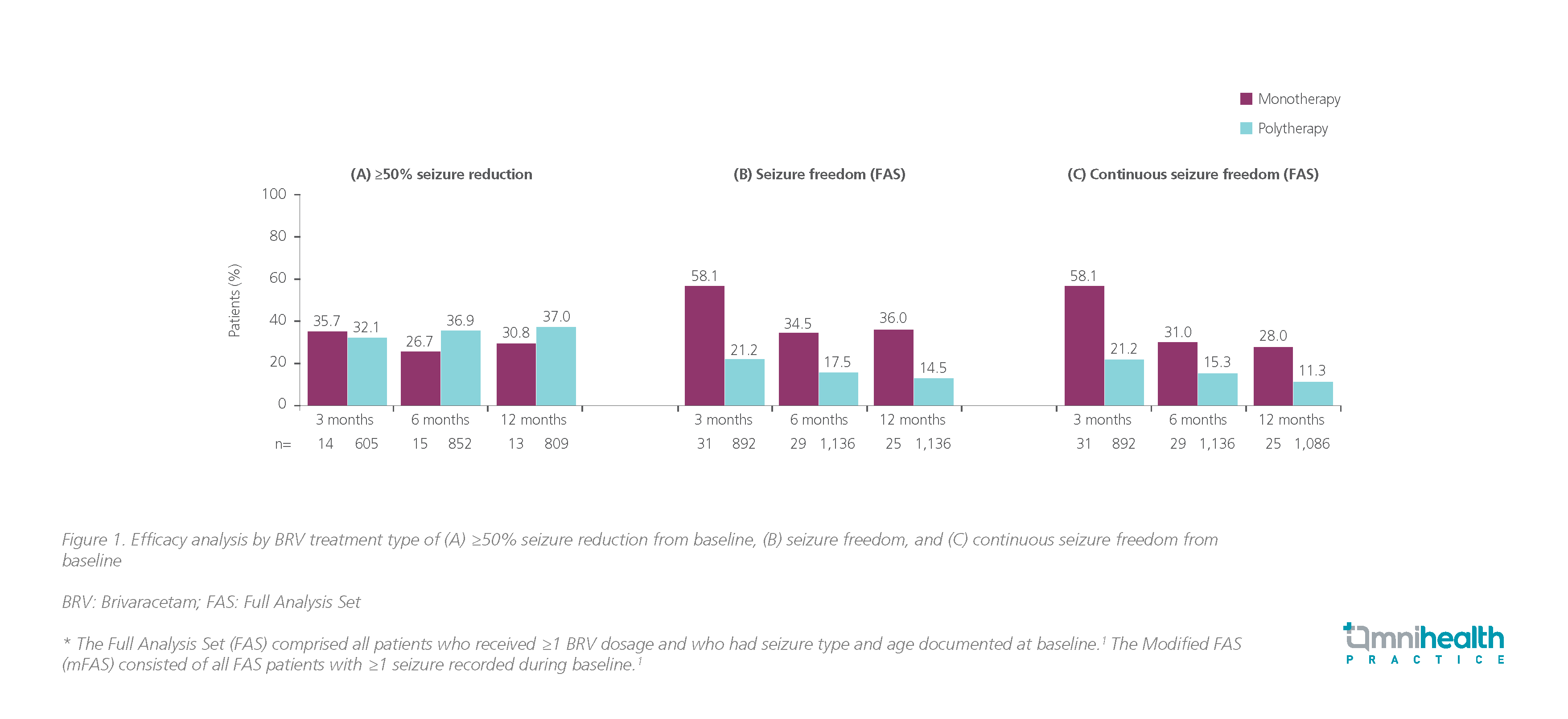
BRV was effective and well tolerated in highly drug-resistant patients
Data in EXPERIENCE/EPD332 came from routine practice in a large international population.3 A total of 1,644 adults from Spain (n=740), Germany (n=488), Australia (n=291), and the US (n=125).3 The median BRV dose was 100mg/day at index (n=1,615) and 200mg/day at 12 months (n=710).3 As evidenced by baseline characteristics, the studied cohorts exhibited a high level of drug resistance.3
According to this pooled analysis, BRV was effective and well tolerated in routine clinical practice, with an 12-month ≥50% seizure reduction of 36.9, continuous seizure freedom of 11.7% and tolerability of 9.3%.3 In the overall BRV treatment population, 32.1%, 36.7% and 36.9% achieved ≥50% seizure reduction at 3, 6 and 12 months respectively; seizure freedom was achieved by 22.4%, 17.9% and 14.9%, and continuous seizure freedom was achieved by 22.4%, 15.7% and 11.7%, respectively (figure 2A).3 Of 1,150 patients with focal-onset seizures and seizure subtype data, 475 (41.3%) had focal-onset seizures without secondary generalization and 675 (58.7%) had focal-onset seizures with secondary generalization.3 Effectiveness outcomes at 3, 6 and 12 months were comparable between the subgroups (≥50% seizure reduction: 31.7% vs. 31.3%, 38.2% vs. 34.6%, and 40.4% vs. 34.0%, respectively; seizure freedom: 14.8% vs. 29.0%, 12.7% vs. 23.5%, and 12.1% vs. 18.1%, respectively; continuous seizure freedom: 14.8% vs. 29.0%, 11.0% vs. 21.3%, and 7.5% vs. 16.7%, respectively) (Figure 2B).3 No new safety concerns were observed. Consistent with prior BRV studies, the 4 most common TEAEs reported by ≥3% of patients at any timepoint were fatigue, dizziness, irritability, and somnolence, demonstrating that BRV was effective and well tolerated even in highly drug-resistant patients.3
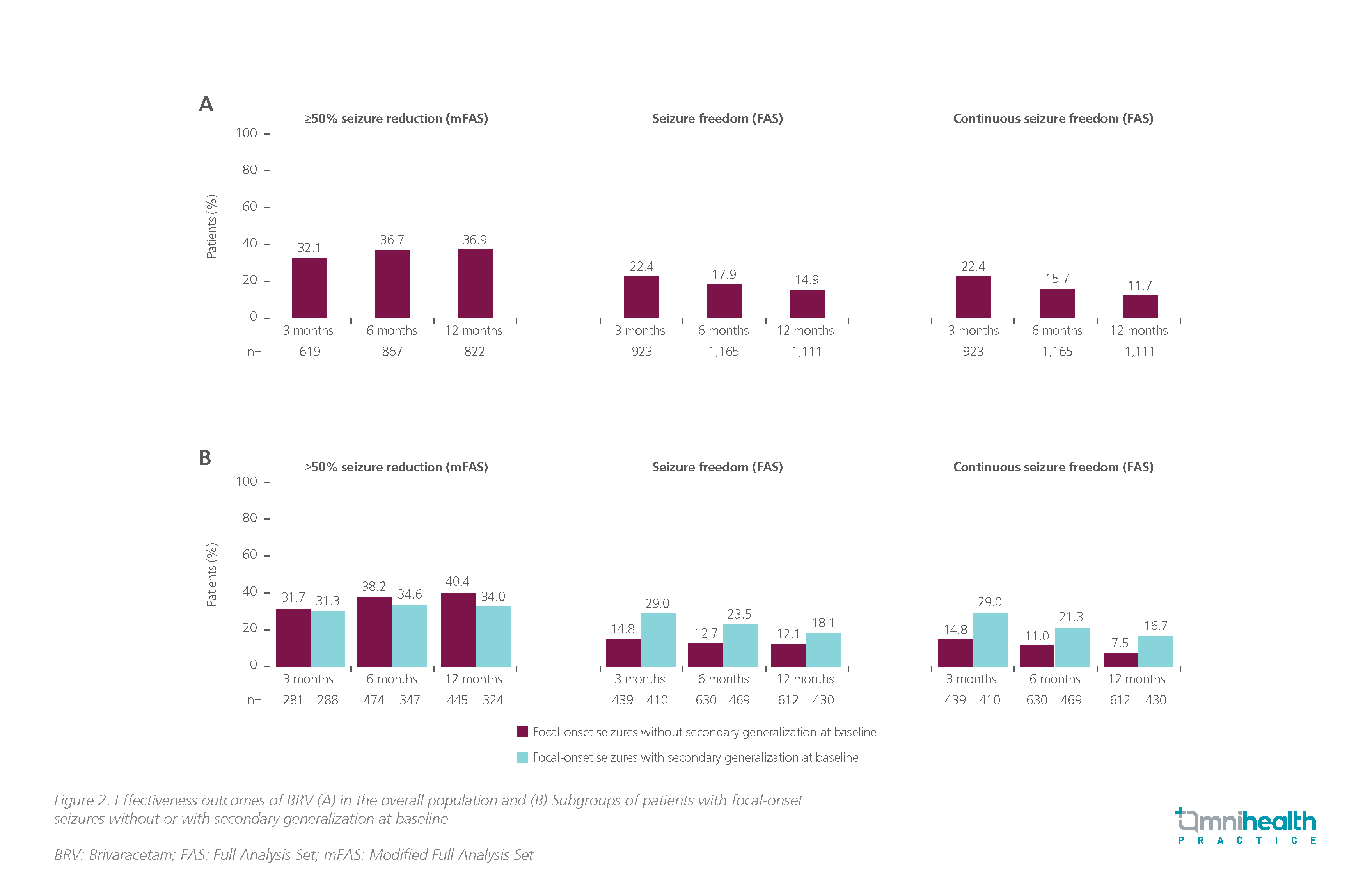
Conclusion
In conclusion, data from EP0077, EP0088, and EXPERIENCE/EPD332 demonstrated that patients with fewer lifetime ASMs upon the initiation of BRV had numerically higher retention and seizure freedom rates, and numerically lower incidence of TEAEs and discontinuations due to either the lack of efficacy or TEAEs.1-3 BRV monotherapy patients had numerically higher seizure freedom and continuous seizure freedom rates with less treatment discontinuation compared with polytherapy patients in the EXPERIENCE/EPD332 study.1,3 The analyses of the 3 studies suggested that BRV was effective and well-tolerated in real-world settings.1-3
|
TWHK-P-BR-EPOS-2300001
Date of Approval: 17/2/2023


