EXPERT INSIGHT
Stride with dual immunotherapy and more: the new and evolving SoC for uHCC management
Multi-kinase inhibitors including sorafenib and lenvatinib were the first-line systemic treatment options for unresectable hepatocellular carcinoma (uHCC) patients, albeit with a median overall survival (OS) of around 1 year.1 Following the regulatory approval based on the significant OS improvement in 2020, atezolizumab + bevacizumab (atezo/bev) became a new and current standard of care (SoC) for uHCC.1 Despite the tremendous advancement, additional systemic treatment options with long-term efficacy and well tolerated safety profiles are still warranted to cater for individual medical needs of uHCC patients.2 Very recently, a novel immunotherapy (IO) combination (i.e., the STRIDE regimen) featuring a single high-priming dose of tremelimumab (TREME) [an anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4)] and every-4-week durvalumab (DUR) (anti-PD-L1) showed encouraging clinical efficacy and high tolerability in a phase 3 trial, becoming the latest addition to the armamentarium of first-line systemic treatment options for uHCC patients.2 In the 4th AGM and HBP workshop organized by the Hong Kong Stereotactic Body Radiation Therapy (SBRT) Study Group, Professor Arndt Vogel and Dr. Chiang, Chi-Leung presented new data and discussed the latest advancement in the rapidly evolving treatment landscape of uHCC. Subsequently in an interview with Omnihealth Practice, Dr. Chiang shared his insights into the new dual IO treatment option in uHCC. He also provided updates on the ongoing study START-FIT [transarterial chemoembolization (TACE) + SBRT + dual IO] with promising early results. At the end of the interview, he shared a clinical case which demonstrated the remarkable efficacy of SBRT + dual IO in a local uHCC patient.
The evolving armamentarium for uHCC over the past 2 decades
Just about 15 years ago, patients diagnosed with uHCC had a dismal prognosis with a median OS of only about 8 months.3 Systemic treatment options were limited to chemotherapy, which had no proven clinical benefits in prolonging patients’ survival.3 Since 2007, there has been increasing number of systemic therapeutic options for treating uHCC, with sorafenib bringing the first breakthrough and subsequently becoming the SoC for uHCC.3 In 2018, given its non-inferior efficacy in OS to sorafenib, lenvatinib was approved as an alternative first-line option for the uHCC treatment.4 Further advance was needed since both first-line treatment options (i.e., lenvatinib and sorafenib) only modestly improved OS and were associated with considerable side effects, such as hypertension, impaired wound healing and diarrhea, which may impair quality of life (QoL) in some patients.4-6
In 2020, atezo/bev demonstrated a significantly improved median OS of 19.2 months vs. 13.4 months with sorafenib (HR=0.66; 95% CI: 0.52-0.85; p=0.0009).7 Based on the positive data, international guidelines such as the European Society of Medical Oncology (ESMO) were updated to recommend atezo/bev as a new SoC in first line systemic therapies for uHCC, while lenvatinib or sorafenib can be considered when the former was contraindicated or not tolerable by patients (figure 1).8 The remarkable survival benefits with atezo/bev showed great promise for further OS improvement in uHCC with IO-based regimen.8 As such, a number of studies evaluating the efficacy of IO in uHCC have been conducted, including the HIMALAYA trial which evaluated the therapeutic effects of DUR + TREME (i.e., dual IO) in uHCC patients.
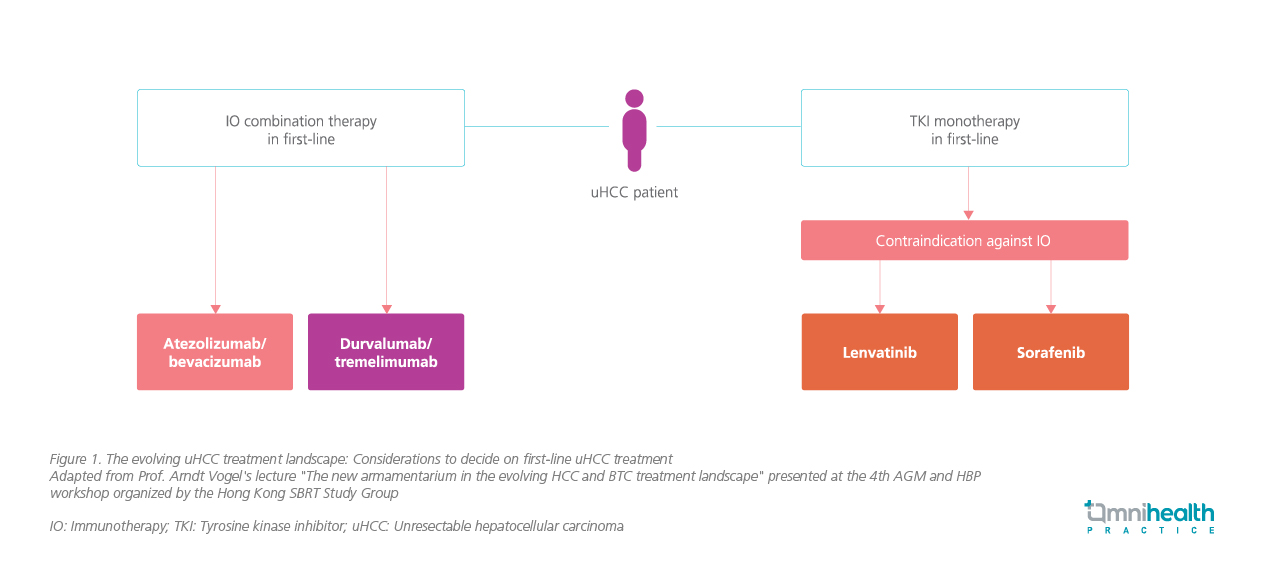
The emerging role of dual blockade by DUR and TREME as first-line uHCC treatment
The underlying mechanism of the dual IOs is based on the synergistic effect obtained from the simultaneous blockade of CTLA-4 and programmed cell death ligand1 (PD-L1).9 CTLA-4 inhibits the priming and activation of lymphocytes early in the antitumor immune response.2 PD-L1 modulates immune responses in the tumor microenvironment, downstream of lymphocyte activation.2 An enhanced antitumor effect may be seen when CTLA-4 and PD-L1 inhibitors are combined, particularly in tumor types for which each monotherapy showed clinical activity, such as in hepatocellular carcinoma and melanoma.2
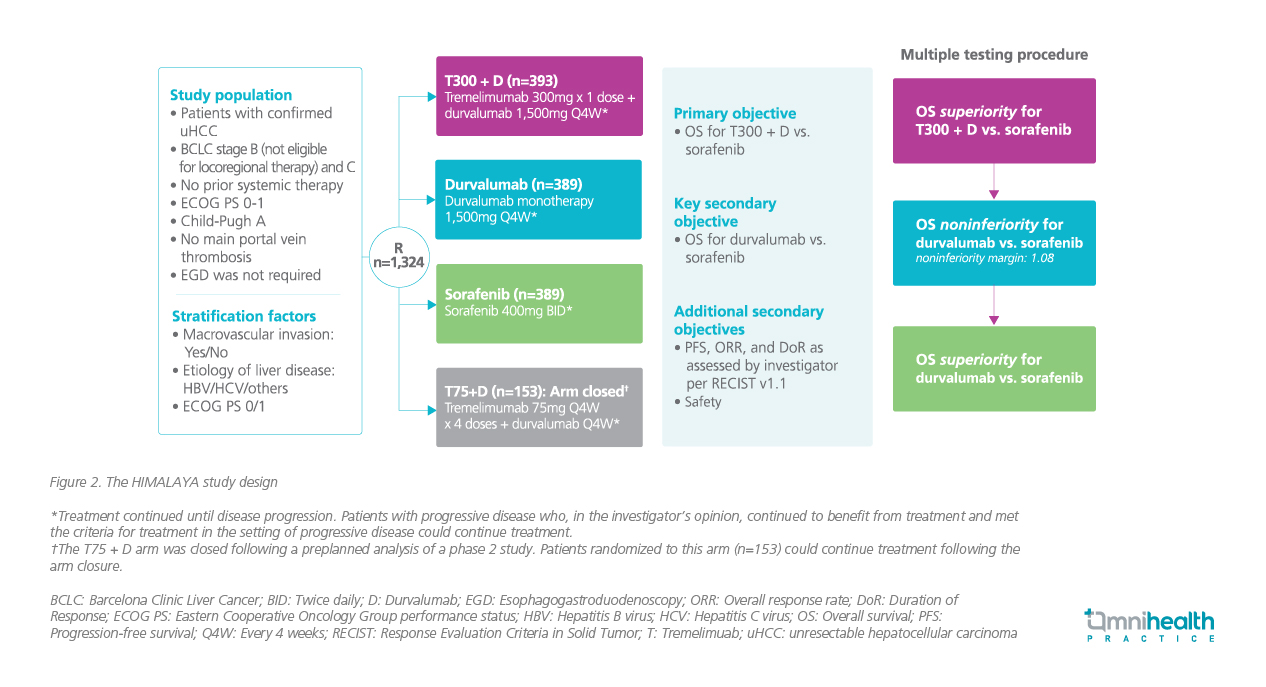
To evaluate the clinical efficacy of DUR + TREME in uHCC, a global, open-label, phase 3 HIMALAYA trial was conducted.2 This trial compared the survival benefits of DUR + a single priming dose of TREME vs. sorafenib among uHCC patients.2 Inclusion criteria for this trial were confirmed uHCC with Barcelona Clinic Liver Cancer (BCLC) stage B (but ineligible for locoregional therapy) or C, with no prior systemic therapy, and a classification of Child-Pugh Score class A.2 A total of 1,171 eligible patients were enrolled and randomized 1:1:1 to receive DUR + TREME, or DUR monotherapy, or sorafenib (figure 2).1
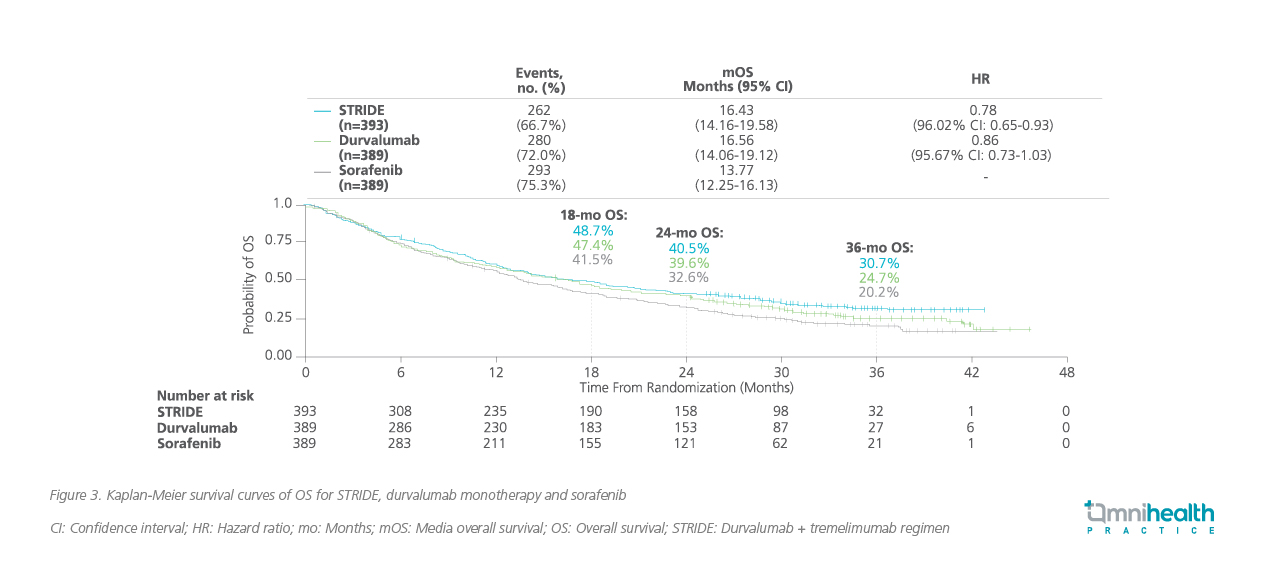
With a median follow-up of 33 months, DUR + TREME demonstrated a superior and prolonged median survival of 16.43 months vs. 13.77 months for sorafenib, with a relative OS risk reduction of 22% (HR=0.78; 96.02% CI: 0.65-0.93; p=0.0035) (figure 3).2 On the other hand, DUR monotherapy was also shown to be non-inferior to sorafenib for OS (median OS 16.56 months vs. 13.77 months; HR=0.86; 95.67% CI: 0.73-1.03).2 The 3-year OS rate was improved with DUR + TREME and DUR monotherapy when compared with sorafenib (30.7% vs. 24.7% vs. 20.2%).2 Of note, the 3-year OS results of the HIMALAYA trial revealed the plateauing of the tail in the Kaplan-Meier survival curves, indicating that patients who responded to DUR + TREME could achieve long-term survival.
In this trial, DUR + TREME showed a well tolerated safety profile with the rates of grade 3 or 4 adverse events (AEs) being comparable to sorafenib (50.5% vs. 52.4%).2 Besides, the addition of a single dose of TREME to DUR, and DUR monotherapy improved median time to deterioration of patient reported global health status or QoL (7.5 months and 7.4 months) when compared with sorafenib (5.7 months).2
In an exploratory analysis of HIMALAYA, consistent OS benefits with DUR + TREME were observed across Child Pugh Score class A patients with albumin-bilirubin (ALBI) grades 1-3.10 Among patients with ABLI grade 1, DUR + TREME achieved a median OS of 23.43 months vs. 19.02 months for sorafenib (HR=0.79; 95% CI: 0.62-1.01).10 For patients with ALBI grade 2/3, DUR + TREME achieved a median OS of 11.30 months vs. 9.72 months with sorafenib (HR=0.83; 95% CI: 0.65-1.05).10 These findings highlighted the retained OS benefits with DUR + TREME among patients with poorer liver function.11
Based on the positive findings, the latest BCLC recommendations include DUR + TREME as an alternative first-line option to atezo/bev for uHCC.11 “The increasing number of frontline treatment options is definitely a great news to our patients since they could be offered the most appropriate therapies catering for their individual needs,” Prof. Vogel said, “In fact, I do look forward to the future advancement in uHCC treatments that can achieve a median OS beyond 20 months.”
Local expert insights into the HIMALAYA trial
In an interview with Omnihealth Practice, Dr. Chiang shared his insights over the new HIMALAYA data and discussed their implications on the local practice. He recalled that just 5 years ago, the uHCC treatment was limited to sorafenib with a slim chance of long-term survival. He agreed that the availability of IO has, in fact, revolutionized the uHCC treatment landscape. Apart from the limitations in OS improvement, tyrosine kinase inhibitors (TKIs) are not appropriate for patients with low platelet count and high bleeding risk. “These patients should be treated with IO-based treatments,” Dr. Chiang stressed.
Data showed that the median OS among uHCC patients treated with atezo/bev was significantly prolonged to about 19 months after a median follow-up of 15.6 months.12 It was the first IO + anti-vascular endothelial growth factor (VEGF) combination approved in the uHCC management, and since 2020, it has been adopted in local practice. However, atezo/bev could be contraindicated in patients with baseline varices arising from portal hypertension associated with liver cirrhosis since these patients are at high risk of bleeding. Therefore, patients must be screened and treated for varices before starting atezo/bev which could be rather challenging in the public sector in Hong Kong. Even after starting the treatment, patients are still required to be monitored for bleeding events regularly.
Looking at the positive 3-year OS data of HIMALAYA, Dr. Chiang reckoned that DUR + TREME will be another promising treatment option for uHCC patients. In cancer management, it is a usual practice to look at the median OS data when referring to survival benefits. In recent years, it has been observed that the tail of the Kaplan-Meier survival curves also provides valuable information about patients’ long-term survival. In HIMALAYA, the plateauing of the tail unique to IO was demonstrated in the Kaplan-Meier survival curves, indicating that patients who responded to DUR + TREME could achieve long-term survival for up to 3 years. This phenomenon was rarely seen in the trials with TKI. Moreover, this novel dual IO consists of a regular administration of DUR and a convenient 1 priming dose of TREME, which is deemed to be more convenient than the regular administration of both atezo and bev and could benefit patients who prefer simplified treatments.
“In my experience, DUR + TREME was highly effective and very well tolerated by my patients”, Dr. Chiang said, “The most common AEs I observed from my patients were mainly hepatic events and diarrhea, but they were manageable.”
Dr. Chiang reminded that careful patient selection for each treatment option remained the key to achieve optimal patient outcomes. For uHCC patients with baseline cirrhosis, varices, and high risk of bleeding, the dual IO with DUR and a single dose of TREME will certainly be a viable first-line treatment option.
The emerging role of SBRT + IO in uHCC
During the lecture in the 4th AGM and HBP Workshop, Dr. Chiang discussed the emerging role of the combination of SBRT + IO in uHCC. Previous evidence has indicated that HCC was associated with inflammation and immunosuppressive microenvironment, and radiation, apart from local tumor control, could induce immunogenic cell death and reprogram the tumor stromal microenvironment against immune evasion mechanisms of cancer (i.e., abscopal effect).13 Therefore, combination of IO with radiation therapy could, theoretically, achieve better local tumor regression and systemic control when compared with single-modality treatment.13
This novel strategy was pioneered by the University of Hong Kong in 2019 and was tested in 5 patients with 3 patients had underlying diseases of hepatitis B and 4 patients had BCLC stage C.13 Encouragingly, the new combination of SBRT + IO resulted in a remarkable response rate with 2 patients achieving complete response by the modified Response Evaluation Criteria in Solid Tumor (mRECIST).13 The median progression-free survival (PFS) was 14.9 months (range: 8.6-19 months).13 In addition, the treatment was well tolerated with grade 3 toxicity occurring in only 1 patient (pneumonitis and skin rash).13
In a later single-arm phase 2 trial (the START-FIT trial), 33 patients with uHCC were enrolled and given a single episode of TACE followed by SBRT 28 days afterward and avelumab 14 days after SBRT.14 The patients were followed up by a median of 17.2 months.14 The objective response rate (ORR) was 62.5% (95% CI: 45.3-77.1) with 43.8% of patients achieving complete response.14 The median OS was 30.3 months (95% CI: 22.7-37.8), and the PFS was 20.7 months (95% CI: 14.6-26.8).14 About 9% of patients were down-staged to curative resection.14
To further the research of multi-modality treatments in uHCC, another phase 2 trial evaluating the efficacy of TACE + SBRT + dual IO of DUR/ TREME (i.e., the START-FIT Double IO trial) was initiated.15 Dr. Chiang expected that similar, positive results can be achieved with the dual IO regimen in this ongoing trial. During the interview with Omnihealth Practice, Dr. Chiang shared a clinical case to illustrate the favorable response and progress of a participating patient who has achieved complete remission for over 10 months with minimal AEs reported.
A case sharing from the ongoing START-FIT Double IO (DUR + TREME) trial
A 70-year-old male patient who had been diagnosed with prostate cancer underwent radical prostatectomy and lymph node dissection in 2013. When he participated in the START-FIT Dual IO trial, he had BCLC stage C1 HCC with Child-Pugh score of A5, ABLI grade 2, ECOG PS of 0, and alpha-fetoprotein (AFP) of 5,007ng/mL. The patient’s initial images of magnetic resonance imaging (MRI) or positron emission tomography/ computed tomography (PET/CT) revealed a centrally located lesion of 13.6cm, suggestive of HCC at S4, 8, 7 with invasion of the middle and right hepatic vein. CT thorax showed no distant metastasis.
TACE was performed in early March 2021, and SBRT was given with 30Gy in 5 fractions in early April 2021, followed by the STRIDE regimen (i.e., 1 priming dose of tremelimumab 300mg + durvalumab 1,500mg every 4 weeks) initiated in late April 2021.
After 2 cycles of STRIDE, the patient’s AFP was normalized, and his MRI images showed complete response by mRECIST which was sustained throughout the treatment course. Also, CT thorax continued to show no distant metastasis.
In total, 6 cycles of STRIDE regimen were given and completed in October 2021. The patient was followed up with regular MRI images every 3 months and CT thorax every 6-9 months. He remained in complete remission as of June 2022. Overall, the patient tolerated the treatments well, except for grade 2 maculopapular rash and grade 1 pruritus which resolved with topical corticosteroid.
Conclusion
All in all, the uHCC treatment landscape continues to evolve, with the dual immunotherapies of DUR and TREME providing a novel, well tolerated and effective alternative first-line treatment in uHCC to cater for the unmet medical needs of patients. Beyond DUR/TREME alone, the trio of TACE, SBRT, and the dual IO showed great promise in phase 2 trials for further outcome improvement among uHCC patients. “Efforts in the scientific community in advancing uHCC treatments continue, and we shall look forward to the upcoming studies and further game-changing advancement in uHCC in the years to come,” Dr. Chiang said at the end of the interview.

