CASE REVIEW
BV consolidation therapy to reinforce CR among high-risk post-auto HSCT patients with HL: A local case sharing
Despite the fact that Hodgkin’s lymphoma (HL) patients generally have very high cure rates of 70%-90% with frontline multi-agent chemotherapies, the battle against this hematological malignancy is still ongoing.1,2 About 20% of HL patients were found failure to stay in complete remission (CR) within a year after the initial frontline therapies, and required autologous hematopoietic stem cell transplantation (HSCT) for the improvement of long-term outcomes.1 Yet, autologous HSCT could only cure 50% of the high-risk patients, meaning that half of them still experience progressive disease after autologous HSCT.2 Enormous efforts have been made to address the unmet medical needs of these patients since the 1990s, but a breakthrough only came in 2015 when brentuximab vedotin (BV) was investigated and shown to be highly efficacious in consolidating CR among high-risk HL patients who had undergone autologous HSCT.2 In an interview with Omnihealth Practice, Dr. Chan, Hiu-Yan Kitty discussed the unmet medical needs of HL patients and presented a case to demonstrate the clinical benefits of BV consolidation therapy in the local clinical setting.
Background
The unmet needs among HL patients with high relapse risk post-autologous HSCT
HL has one of the highest cure rates of all human cancers.1 Approximately 90% of patients with early- or intermediate-stage disease achieve long-term disease-free survival with the frontline doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) protocol.1,2 For patients with early diseases and unfavorable risk factors or more advanced diseases, more intense treatment protocols, such as escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), may be required to obtain a higher initial remission rate.1 Yet, about 20% of patients in the early disease stage and 35%-40% of patients with advanced disease still experience relapse or disease progression after the initial frontline treatments.1 Among these patients, it is well known that patients who have relapsed within 12 months after the initial therapies will not have good survival with other conventional chemotherapies.1 While exploring other alternatives, the approach of salvage chemotherapy followed by autologous HSCT was found to deliver positive results in randomized controlled trials and therefore has been widely adopted in clinical practice to improve the survival of these patients.2 Unfortunately, about 50% of the high-risk patients still experience disease progression following autologous HSCT.2
The emergence of BV in post-autologous HSCT setting
The unmet medical needs among high-risk HL patients have triggered immense efforts to develop consolidation therapies for improving the post-autologous HSCT outcomes since 1990, but the results of most clinical studies were unsatisfactory.2 The consolidation strategies, which have been attempted in the previous studies over the past 20 years, were mainly limited by the difficulties in delivering effective and well tolerated therapy early to patients after autologous HSCT.3 Then, there came a breakthrough in 2015 when the initial results of the AETHERA trial were published, demonstrating remarkably high response rates and a significant improvement in progression-free survival (PFS) with BV consolidation therapy, an antibody-drug conjugate (ADC) consisting of an anti-CD30 antibody (brentuximab) and monomethyl auristatin E (vedotin), among patients with relapsed/refractory HL and a high risk of post-autologous HSCT relapse (vs. placebo).3
Based on the positive trial results, Dr. Chan adopted the BV consolidation therapy in her practice and shared a clinical case to demonstrate the clinical benefits of BV treatment in reinforcing CR after autologous-HSCT.
Case sharing
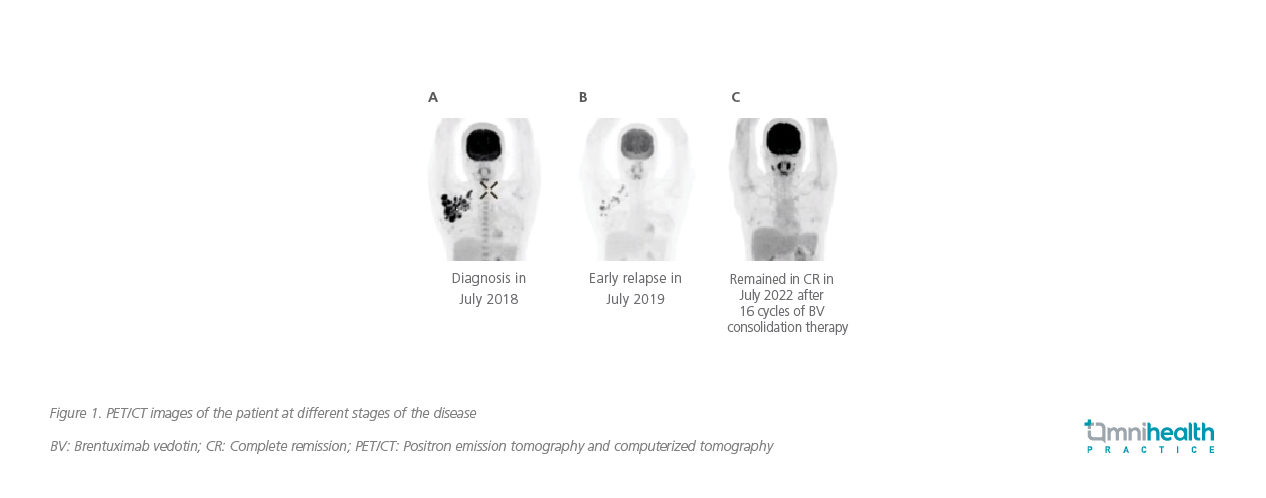
A 37-year-old female without past medical history or family history of hematological malignancy presented with right axillary swelling for 6 months and sought medical attention in mid-2018. Lymph node biopsy was performed in July 2018, and a diagnosis of HL belonging to the most common nodular sclerosis subtype was confirmed. Further examination by positron emission tomography and computed tomography (PET/CT) revealed the involvement of axillary and cervical lymph nodes, thus classifying her condition as stage IIA disease since she did not present with B symptoms (figure 1A). Besides, blood tests showed a highly elevated level of erythrocyte sedimentation rate (ESR) of 61mL/hour, rendering the patient’s prognosis unfavorable. Bone marrow examination showed no marrow involvement in this patient.
The patient was initiated with the frontline ABVD regimen soon after diagnosis. She achieved partial response after 2 cycles of ABVD, with some lymph node involvement remaining on the right axilla with Deauville score of 4. Although her response to ABVD was not optimal, the patient and clinician decided to proceed further cycles of ABVD, avoiding more intensive BEACOPP regimen which is associated with an increased risk of infertility among young women. The patient completed 6 cycles of ABVD in January 2019, but unfortunately, the PET/CT scan was still positive on the right axillary lymph nodes. In July 2019, disease progression was noted as the right axillary region lymph node involvement increased in size and number. Right supraclavicular lymph node biopsy was then performed and an early disease relapse with CD30+ nodular sclerosis subtype HL was confirmed (figure 1B).
The dexamethasone, high-dose cytarabine, and cisplatin (DHAP) regimen was given as salvage chemotherapy to bridge the patient for autologous HSCT. After 2 cycles of DHAP, the size and number of her lymph nodes were significantly reduced, but CR was not achieved. Despite that, autologous HSCT with the combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning proceeded in February 2020.
Three months after autologous HSCT, PET/CT scan showed that the previously enlarged hypermetabolic right axillary lymph nodes were resolved. Since the patient had a high risk of early relapse, BV consolidation therapy at 1.8mg/kg every 21 days for 16 cycles was prescribed in June 2020 after autologous HSCT. Throughout the consolidation treatment, the patient only experienced grade 1-2 sensory type peripheral neuropathy and mild numbness, having no effects on her daily functioning, and was completely resolved after finishing the treatment course.
Overall, the BV consolidation therapy was well tolerated by this patient, and no dose reduction was needed. As of the last follow-up in late July 2022, the patient has remained in CR and continued her fulfilling life (figure 1C).
Discussion
AETHERA was a global phase 3 randomized, placebo-controlled trial designed to assess the efficacy of BV as a consolidation therapy among adult HL patients, who were naïve to BV before autologous HSCT and were at high risk of relapse or progression after autologous HSCT.3 The risk factors for post-autologous HSCT relapses included in this trial were (1) failure to achieve CR with frontline chemotherapy; (2) occurrence of relapses within 12 months from the end of frontline therapy; and (3) extranodal involvement at the start of pre-autologous HSCT salvage chemotherapy.3 A total of 329 eligible patients were enrolled and randomized 1:1 to receive BV 1.8mg/kg (n=165) or placebo (n=164) every 3 weeks for up to 16 cycles, starting 30-45 days after autologous HSCT.3 The primary endpoint was PFS assessed by independent review.3
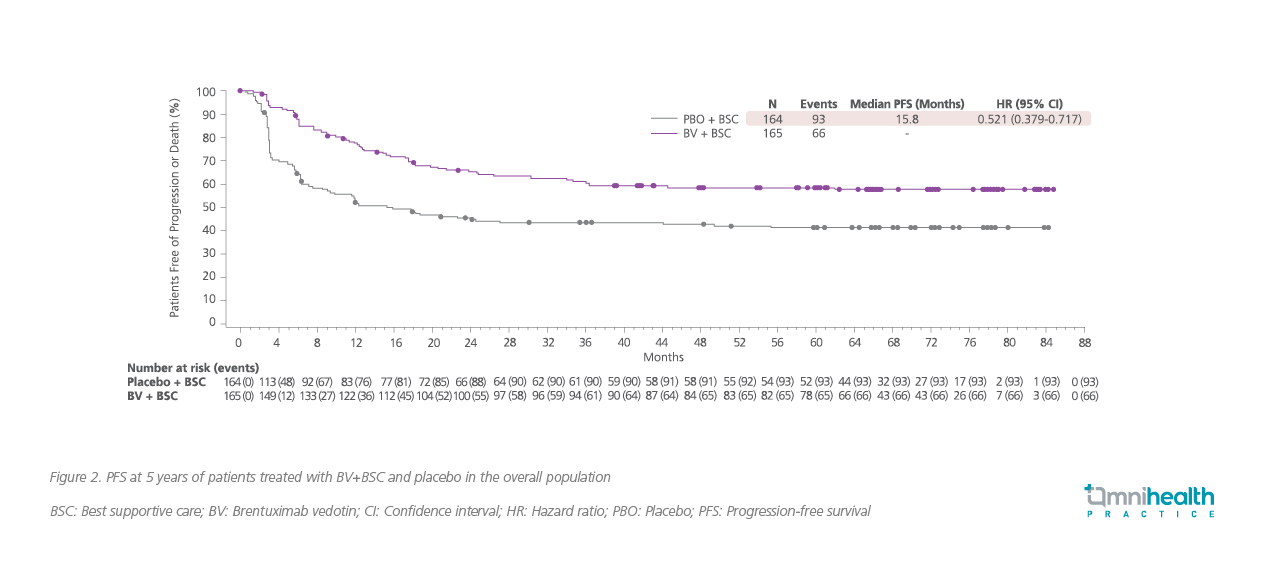
In the 5-year follow-up, patients treated with BV consolidation therapy continued to experience significantly longer PFS vs. placebo (median PFS: Not reached vs. 15.8 months).4 The BV consolidation therapy achieved a significantly longer 5-year PFS rate of 59% (95% CI: 51%-66%) vs. 41% (95% CI: 33%-49%) in the placebo group, with a relative risk reduction in PFS of 47.9% (HR=0.521; 95% CI: 0.379-0.717) (figure 2).4 Among patients with additional pre-autologous HSCT risk factors, the PFS benefits with BV consolidation were more pronounced, reducing the 5-year progression risk by 57.6% (HR=0.424; 95% CI: 0.302-0.596) in patients with ≥2 risk factors; and by 61.0% (HR=0.390; 95% CI: 0.255-0.596) among those with ≥3 risk factors (figure 3).4 In addition, significantly fewer patients in the BV group required subsequent anticancer therapy vs. patients treated with placebo (32% vs. 54%; p<0.0001).4 Of note, for patients in the placebo group who required subsequent lines of treatments, 87% of them received a single-agent BV consolidation therapy.4

Regarding safety, the initial results of the AETHERA trial showed that about 56% of patients treated with BV reported peripheral neuropathy vs. 16% in the placebo group.3 In the 5-year follow-up, 90% of patients who experienced peripheral neuropathy in the BV group reported resolution or improvement. Among them, 73% had achieved complete resolution.4 The median time to resolution or improvement of peripheral neuropathy in the BV group was 37.6 weeks vs. 9.1 weeks in the placebo group.
Overall, the long-term follow-up of the AETHERA trial demonstrated that the BV consolidation therapy was an efficacious and well tolerated treatment option for post-autologous HSCT patients with HL and at high relapse risks.
Dr. Chan remarked that the clinical benefits demonstrated in the AETHERA trial were replicable in clinical practice and was satisfied with the treatment outcomes achieved by the BV consolidation therapy in her patient. Since June 2020, the patient has been in CR for more than 2 years. As this patient has 2 risk factors for refractory disease post-auto-HSCT (i.e., relapsed within 12 months after frontline treatment and only achieved partial response with salvage DHAP therapy), the BV consolidation therapy was expected to provide a greater PFS benefit to this patient when compared to patients with fewer risk factors, according to the trial results. Throughout the previous 2-year treatment course, this patient experienced mild peripheral neuropathy and was entirely resolved soon after the completion of 16-cycle therapy, which was also consistent with the trial’s safety findings.
“The BV consolidation therapy is a promising treatment option for high-risk HL patients to enhance their cure rates after autologous HSCT,” Dr. Chan concluded. “However, the unmet needs remain for patients who have received the BV treatment prior to autologous HSCT, and more treatment options will be required in the future,” Dr. Chan reminded.
Conclusion
In summary, about 20% of HL patients will have refractory diseases, but autologous HSCT can only cure about 50% of these patients. Now, a novel treatment option with proven efficacy and safety has emerged, it is important for clinicians to consider initiating the BV consolidation therapy in the high-risk post-autologous HSCT patients to achieve higher remission rates. Despite the recent advances, the fight against HL continues since the unmet needs remain for high-risk patients who have received BV before autologous HSCT, and alternatives with high efficacy in maintaining CR in these post-transplantation HL patients are warranted.

