CASE REVIEW
A local case sharing: the emergence of BV-CHP as a breakthrough frontline treatment for sALCL
Systemic anaplastic large cell lymphoma (sALCL) belongs to the group of peripheral T-cell lymphomas (PTCLs) characterized by strong and universal expression of CD30.1 It is classified into anaplastic lymphoma kinase (ALK)-negative or -positive subtypes based on the expression of ALK protein.1 Until recently, the standard frontline therapy for sALCL remains CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone)- based regimens, which has high rates of disease relapse despite the modest improvement in survival outcomes, particularly among ALK-negative patients.1 With the advent of the novel regimen BV-CHP (brentuximab vedotin, cyclophosphamide, doxorubicin, prednisolone), the survival outcomes of sALCL patients have improved significantly. As such, international oncology guidelines, such as the National Comprehensive Cancer Network (NCCN), recommend the BV-CHP regimen to replace the CHOP-based treatments as a new standard frontline therapy for sALCL.2 In an interview with Omnihealth Practice, Dr. Kong, Shun-Yin Jacky presented a case to demonstrate the effectiveness and safety of BV-CHP in local clinical settings and discussed the positive impact of BV-CHP on the overall sALCL management.
Background
The unmet needs among sALCL patients
Anaplastic large cell lymphoma (ALCL) is one of the most common subtypes of PTCLs and has some unique pathological features, including the presence of large pleomorphic neoplastic cells and strong expression of CD30.1,3 Compared with the localized forms of ALCL, particularly primary cutaneous ALCLs, sALCL is more aggressive and often warrants a more proactive approach of clinical management and the use of systemic therapy.3 In 2008, the World Health Organization (WHO) divided sALCL into 2 distinct entities, namely ALK-positive and ALK-negative sALCL, based on their different clinical behaviors and outcomes.3 ALK-negative sALCL carries worse clinical outcomes with a 5-year overall survival (OS) rate of about 50% vs. about 70% for ALK-positive sALCL due to a poorer understanding of the ALK-negative subtype and the lack of the available therapeutic agents.3
The CHOP-based regimens belong to conventional chemotherapy and have been the standard of care for frontline sALCL management for decades, although the recommendation was not based on randomized data and the clinical outcomes were suboptimal.3,4 The International Peripheral T cell Lymphoma Project reported that the traditional therapy had delivered low 5-year OS rates of 70%-81% and 42%-49%, and 5-year progression-free survival (PFS) rates of 60% and 36% for patients with ALK-positive and ALK-negative sALCL, respectively, indicating the unmet needs for further improvement in patient outcomes, especially for patients with the ALK-negative subtype.4 In addition, disease relapse and treatment failure with conventional multiagent chemotherapies remain common.1 Over the years, more intensive approaches have been evaluated for prolonging survival in sALCL patients.4 Unfortunately, such efforts failed to pay off, and no significant improvements to the historical CHOP results were obtained.4
Emergence of ADC and a novel CHOP-like regimen
Given that sALCL is characterized by 100% CD30 antigen cell surface receptor expression regardless of the disease stage, line of therapy and transplant status, it is conceivable that novel therapies designated to target this protein would be of high likelihood to bring about a notable improvement in the overall treatment outcomes among all sALCL patients.4,5 Brentuximab vedotin (BV) is an antibody-drug conjugate (ADC) that selectively binds to CD30-expressing tumor cells.5 The delivery of vedotin, an antineoplastic agent, to the cancerous cells upon the antibody binding results in apoptotic cell death of the tumor.5 It was first approved by the United States (US) Food and Drug Administration (FDA) and was utilized in the sALCL management as a monotherapy in the relapsed setting in 2011.3 More recently, the therapeutic effect of BV, in combination with cyclophosphamide, doxorubicin, prednisolone (CHP), has also been investigated in untreated sALCL patients (i.e., as a first-line therapy) in the ECHELON-2 study, a global randomized phase 3 trial.4 With the demonstrated superior survival benefits among sALCL population regardless of the ALK status, FDA and the European Medicine Agency (EMA) approved BV-CHP for the sALCL management in 2019 and 2020, respectively.4 Furthermore, the NCCN guidelines recommend BV-CHP as the only preferred frontline treatment option for ALCL patients.2
Dr. Kong welcomed the approval of the BV-CHP regimen in Hong Kong and adopted this novel CHOP-like regimen in his clinical practice with efficacious and satisfactory clinical outcomes.
Case sharing
A man in his 30s presented with B symptoms in April 2019 and was later diagnosed with stage IV ALK-positive sALCL. He had neither comorbidities nor a family history of PTCLs. The complete blood count (CBC), blood biochemistry, positron emission tomography and computerized tomography (PET-CT) were performed in the initial diagnostic workup. The CBC results showed elevated levels of white blood cell (WBC) count of 48.2x109/L and platelet count of 381x109/L, and a decreased level of hemoglobin of 7.9g/dL. The blood biochemistry found increased levels of serum urea level of 11.6mmol/L and lactate dehydrogenase (LDH) level of 590U/L, implying injuries of the kidney and liver. The PET-CT scans from the skull vertex to the upper thigh were obtained, with hypermetabolic lymphadenopathy observed in the cervical, thoracic, abdominal and pelvic regions, which were highly suggestive of lymphomas (figure 1).
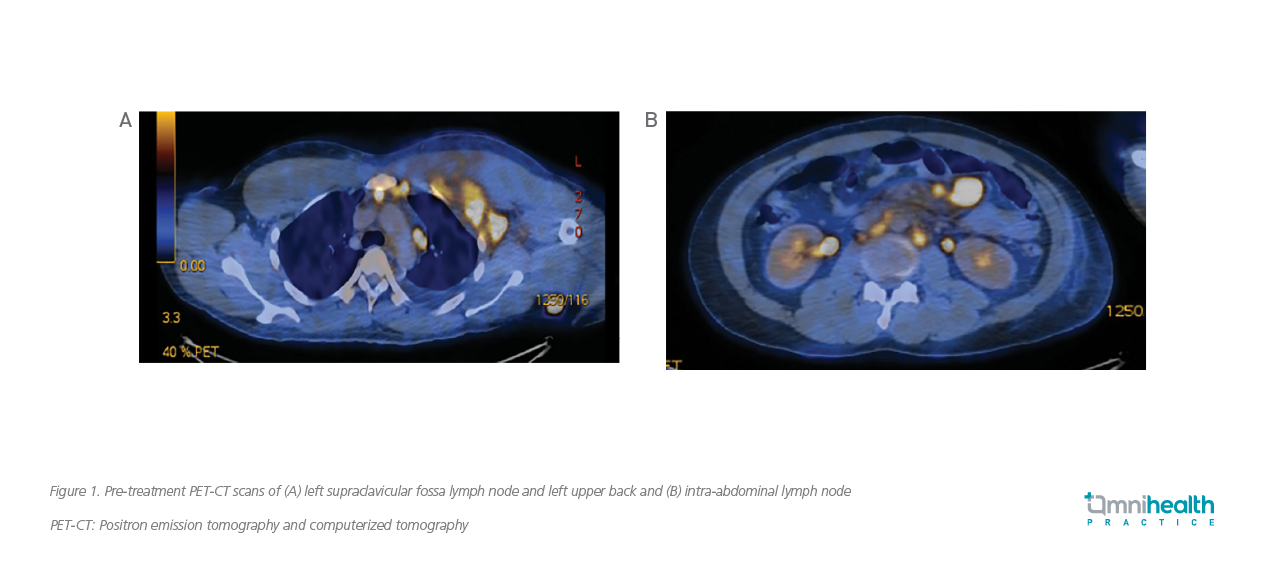
The patient then started on a 6-cycle BV-CHP regimen soon after confirmation of sALCL diagnosis in April 2019. He responded well to the prescribed treatment with his B symptoms subsided quickly upon completion of the first 2 cycles of BV-CHP. His WBC was reduced from the very high level of 48.2x109/L to 4.6x109/L, and the hemoglobin level also increased significantly from 7.9g/dL to 9.4g/dL, although the platelet count remained high at 571x109/L. Remarkable improvements in serum urea and LDH levels were also observed. The serum urea level significantly dropped to 2.7mmol/L, and the serum LDH level had normalized to 198U/L, suggesting the rapid renal and hepatic recoveries with the BV-CHP treatment.
Upon completion of the 4th cycle of BV-CHP, the patient’s condition continued to improve. His WBC (5.5x109/L) remained within the normal range, and his hemoglobin level increased further to 12.2g/dL. Moreover, the platelet count went down significantly to 268x109/L and returned to the normal level.
The patient completed his 6th cycle of BV-CHP treatment in November 2019 with remarkable clinical outcomes. All his blood and biochemical parameters had returned to the normal range. The PET-CT scans were obtained again after completion of the treatment course. All previously hypermetabolic lymphadenopathies in cervical, thoracic, abdominal, and pelvic regions had either normalized or resolved (figure 2). No more hypermetabolic lymphadenopathy or lesion suggestive of lymphomatous disease was detected.
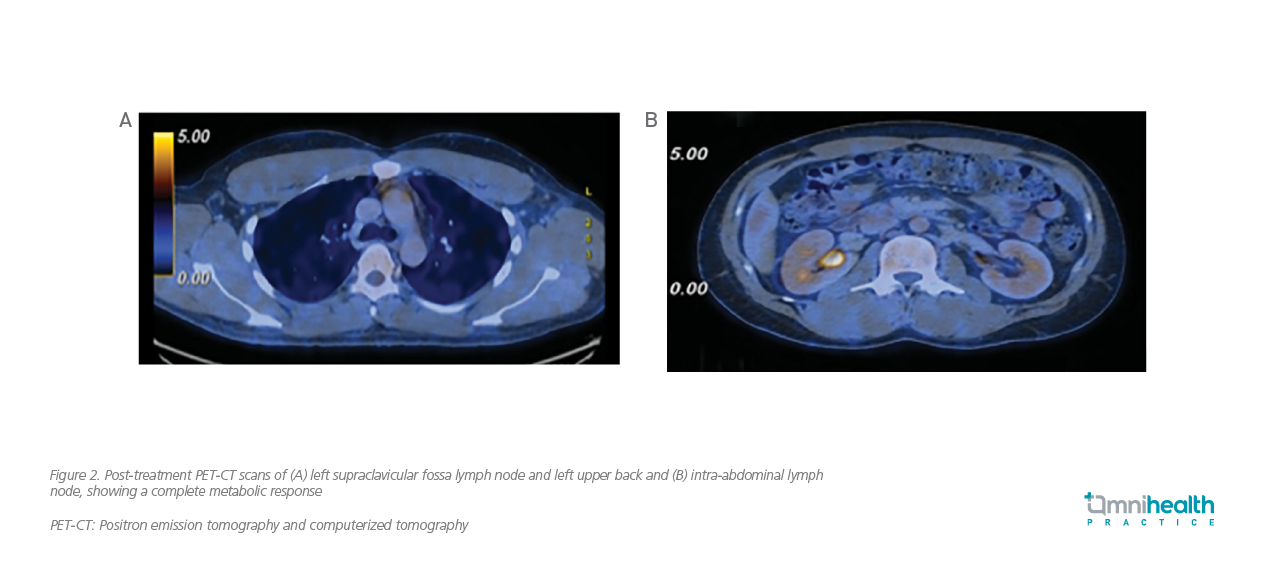
Overall, BV-CHP was well tolerated by this patient, and no serious adverse event (AE) was reported. His quality of life (QoL) was maintained throughout the 6 cycles of BV-CHP regimen. Besides, he was able to quickly resume working and live a normal life. As of January 2022, this patient has been staying in remission for more than 2 years.
Discussion
The global approvals and international recommendations of the BV-CHP regimen as a frontline therapy for ACLC patients were based on its clinical efficacy demonstrated in the double-blind, randomized, phase 3 ECHELON-2 study, which aimed to compare PFS achieved by the BV-CHP and the standard CHOP regimens among patients with previously untreated CD30-positive PTCL in 1:1 randomization.6 In this study, a total of 452 patients were enrolled, with 70% of the patients (n=316) diagnosed with sALCL. Among the ALCL patients, 48% had ALK-negative subtype (n=218) and 22% had ALK-positive (n=98).6 In addition, most of the included patients had advanced disease (stage III: 27%; stage IV: 53%) and poor prognosis (International Prognostic Index ≥2: 78%) at baseline.6
In a recent 5-year update of ECHELON-2 (median follow-up: 47.6 months), the BV-CHP group achieved a 5-year PFS rate of 51.4% (95% CI: 42.8%-59.4%) vs. 43.0% (95% CI: 35.8%-50.0%) by the CHOP group in the overall population (HR=0.70; 95% CI: 0.53-0.91; p=0.0077).7 The median PFS for the BV-CHP group was found to be 62.3 months (95% CI: 42.0 months-not evaluable) vs. 23.8 months (95% CI: 13.6-60.8 months) in the CHOP group.7
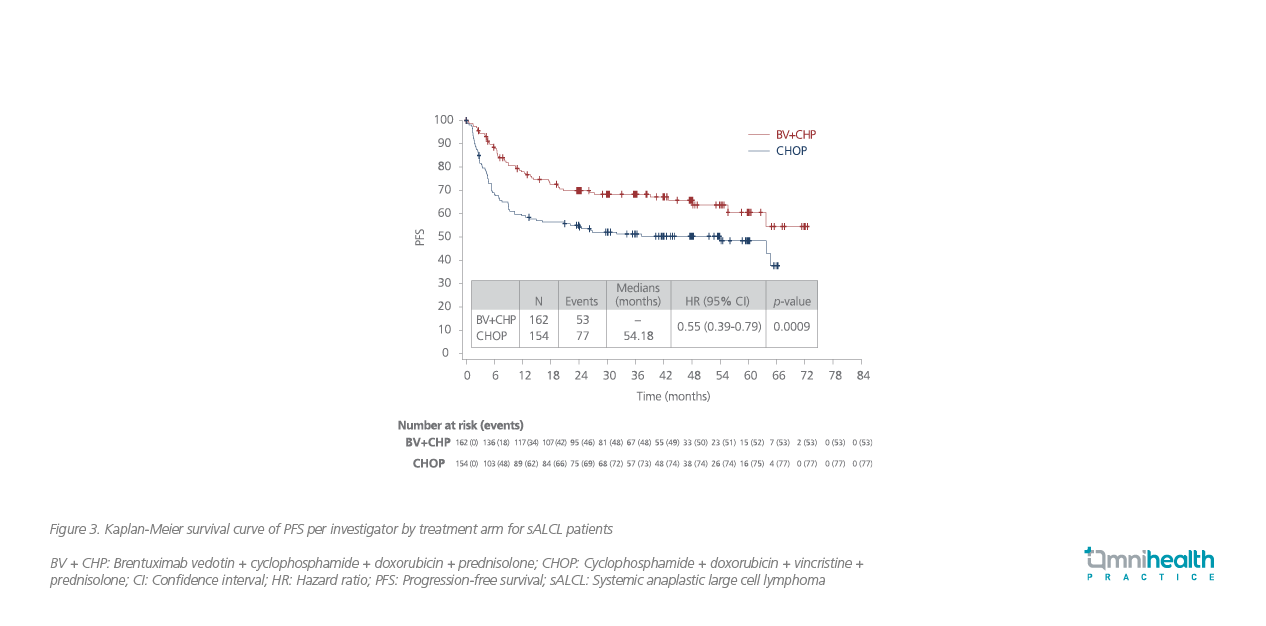
In the subgroup of sALCL patients, about 60.6% of patients remained progression-free after 5 years of the BV-CHP treatment (95% CI: 49.5%-69.9%) compared with 48.4% in the CHOP group (95% CI: 39.6% to 56.7%) (HR=0.55; 95% CI: 0.39-0.79; p=0.0009) (figure 3).7 Similar PFS benefits with the BV-CHP treatment were observed across different subgroups of patients, including those with ALK-positive and ALK-negative sALCL (figure 4).7
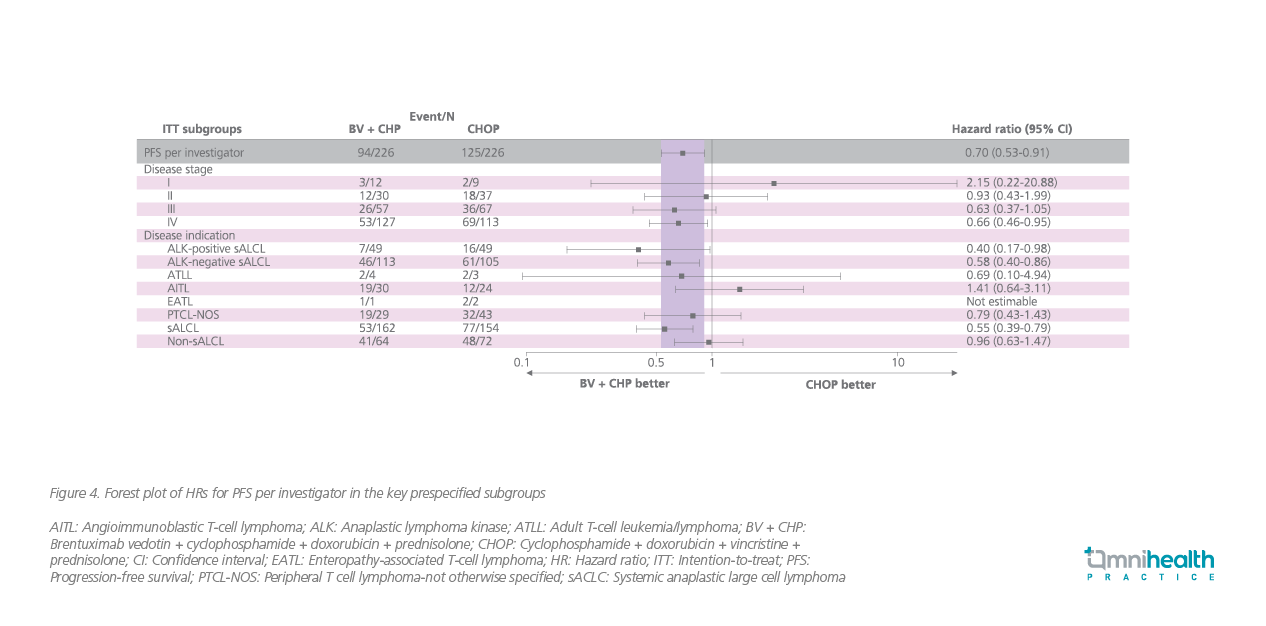
Furthermore, the ECHELON-2 study also evaluated the response rate of sACLC patients who had relapsed after the frontline therapy to the BV-CHP retreatment.7 Among the 19 relapsed patients who were re-treated with BV-CHP, 12 patients obtained a treatment response (objective response rate: 63%), and 8 patients (42%) achieved complete remission.7
Peripheral neuropathy (PN) was the most reported AE and occurred in about 50% of patients in both treatment arms.7 PN in more than 70% of patients was improved or resolved over time.7
Dr. Kong stressed that compared with the conventional one-size-fits-all multiagent chemotherapies, particularly the CHOP-based regimens which of ten have poor survival outcomes in sALCL patients, the novel BV-CHP was designed to target CD30-expressing tumor cells and was proven by clinical trials with a significantly better PFS and a high rate of complete remission in patients. “In this molecular era, targeted therapy is much needed, and the novel BV-CHP regimen is a game-changer in the treatment landscape of sACLC with significant survival improvements,” stated Dr. Kong.
High efficacy aside, BV-CHP also has the advantage of reducing the risk of overlapping neurotoxicity that could have been worsened by using 2 microtubule-disrupting drugs in the CHOP-based regimens. The avoidance of another anti-microtubule agent in the BV-CHP regimen could be conducive to maintaining patients’ QoL throughout the treatment course and the ultimate treatment success.
“The BV-CHP regimen has a predictable and manageable safety profile,” Dr. Kong highlighted. Therefore, the hematological toxicity associated with the BV-CHP treatment can be monitored closely to allow for early and timely intervention. Possible AE management approaches include dose interruption and the use of granulocyte colony-stimulating factor (G-CSF), if needed, depending on the severity of neutropenia. PN can also be managed through dose interruption or modification in case of occurrence.
Conclusion
The novel BV-CHP regimen has demonstrated significant improvements to the historical CHOP results and brought the long-awaited advancement in sALCL management. Patients are now offered a more efficacious alternative with a predictable and manageable safety profile to bring the disease under control while maintaining a good QoL. Wider adoption of BV-CHP in the local clinical practice will certainly benefit more patients and help them resume a normal and fulfilling life

