EXPERT INSIGHT
Carfilzomib: Expert insight into the Asian real-world data and a local case sharing
Carfilzomib-based therapies have been approved for the management of patients with relapsed/refractory multiple myeloma (rrMM) based on the positive results from 2 randomized clinical trials, namely ASPIRE and ENDEAVOR.1-5 Although the 2 trials showed remarkable clinical benefits of carfilzomib-based rrMM treatments, it was still unclear whether these results could translate into routine clinical practice since some patients might not be eligible for the trials. As such, a prospective real-world (RW) study was performed to evaluate the effectiveness of carfilzomib + dexamethasone (Kd) or carfilzomib + lenalidomide + dexamethasone (KRd) in the RW setting among the Asian population, hoping to provide additional insights into the efficacy-effectiveness gap.6 In an interview with Omnihealth Practice, Dr. Kong, Shun-yin Jacky shared his insight into the latest Asian RW data of carfilzomib and presented a clinical case to demonstrate the effectiveness of Kd in rrMM management in the local clinical setting.
ASPIRE and ENDEAVOR: Superior PFS and OS benefits of KRd and Kd in rrMM
ASPIRE was a randomized, placebo-controlled phase 3 trial which enrolled 792 rrMM patients and were randomized 1:1 to receive either carfilzomib with KRd or lenalidomide + dexamethasone (Rd).1 The median age in the carfilzomib group was 64 years, and the proportion of patients with high cytogenic risk was low (12.1%).1 Patients treated with KRd achieved a median progression-free survival (PFS) of 26.3 months vs. 17.6 months in the Rd group (HR=0.69; 95% CI: 0.57-0.83; p=0.0001), and had a median overall survival (OS) of 48.3 months vs. 40.4 months in the Rd group (HR=0.79; 95% CI: 0.67-0.95; pone-sided =0.0045) (figure 1).1,2
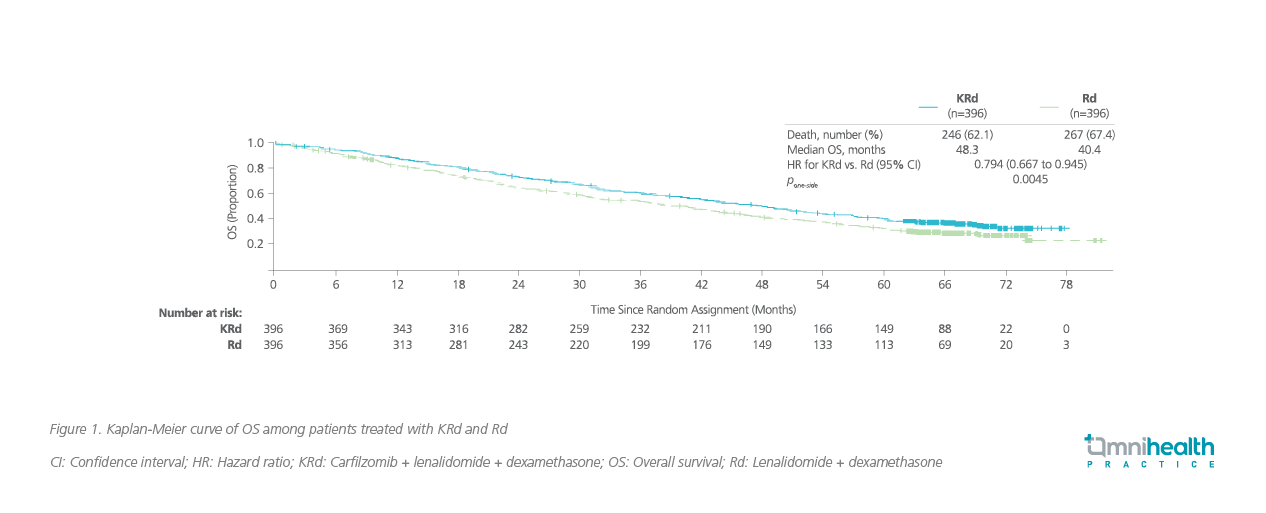
In the ENDEAVOR trial, 929 rrMM patients with prior 1-3 previous treatments were recruited and randomized 1:1 to receive Kd or bortezomib and dexamethasone (Vd).3 Similar to the ASPIRE trial, the median age of patients in the carfilzomib group was 65 years, and few patients had high cytogenic risk (21%). Patients treated with Kd achieved a median PFS of 18.7 months vs. 9.4 months in the Vd group (HR=0.53; 95% CI: 0.44-0.65; p<0.0001) and had a median OS of 47.6 months vs. 40.0 months in the Vd group (HR=0.79; 95% CI: 0.648-0.964; pone-side=0.010) (figure 2).3,4 In an Asian subgroup analysis of ENDEAVOR, the survival benefits of the Kd regimen remained more prominent when compared with the Vd group, achieving a longer median PFS (14.9 months vs. 8.8 months; HR=0.599) and longer median OS (47.6 months vs. 38.8 months; HR=0.856).5
The ongoing Asian RW study of carfilzomib
To confirm the RW effectiveness of carfilzomib-based regimen in the Asian rrMM population, a prospective, single-arm cohort study was initiated in 2019 and is still ongoing.6 rrMM patients in the Asia-Pacific region who had received ≥1 dose of carfilzomib and had ≥1 prior line of multiple myeloma (MM) treatment before carfilzomib-based treatments are eligible for evaluation.6 About 300 patients are anticipated to be included, and the last patient record for analysis will be collected by the first quarter of 2023.6
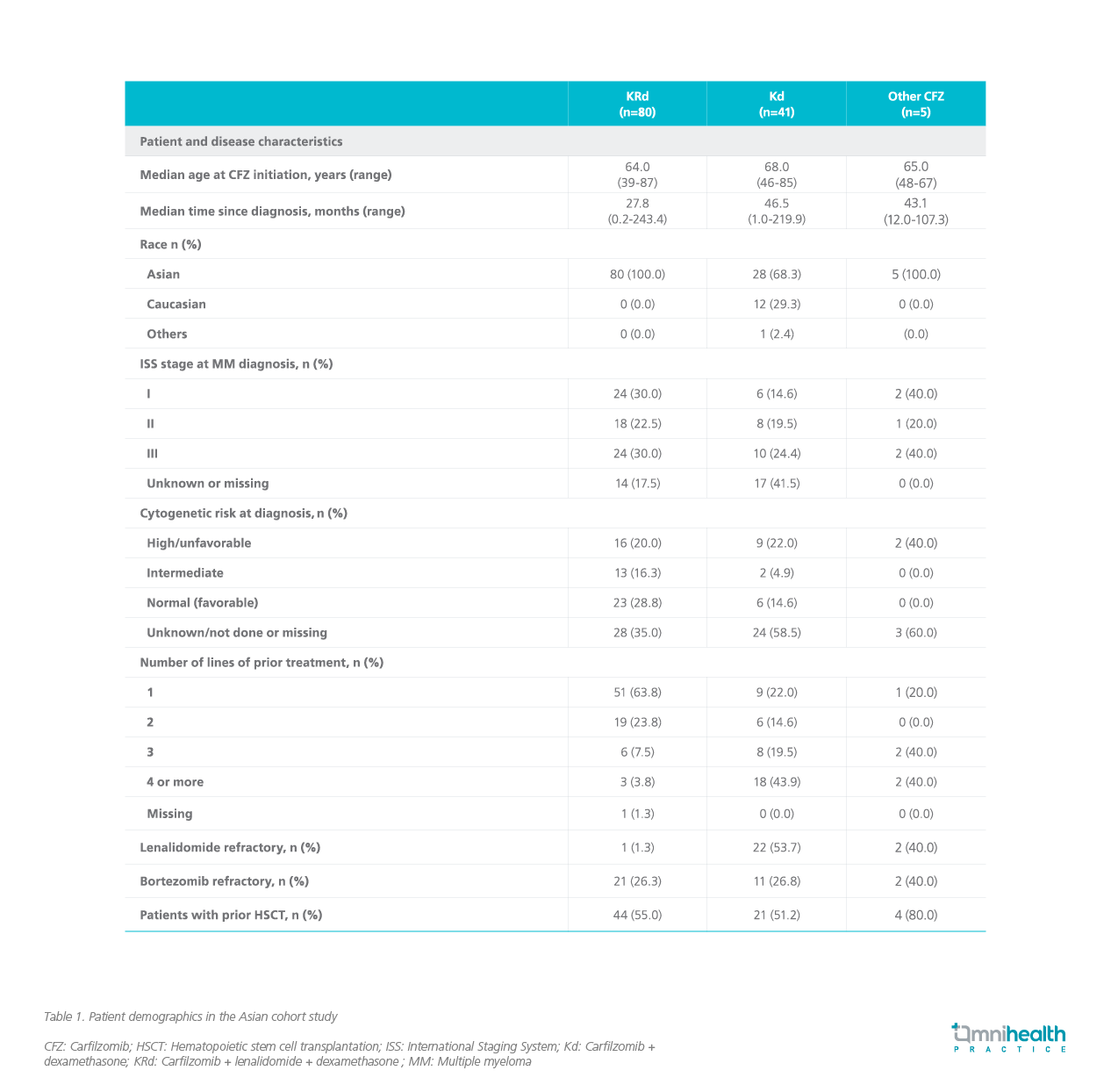
As of September 2020, some 126 patients have been recruited from 5 regions/countries, including Hong Kong.6 Among them, 63% and 33% were prescribed with KRd and Kd, respectively.6 Noteworthy is that the proportion of high-risk patients was similar in this Asian cohort study to those recruited in the clinical trials, and the proportion of bortezomib-refractory patients in this cohort study was also closer to those in the trials (table 1).6
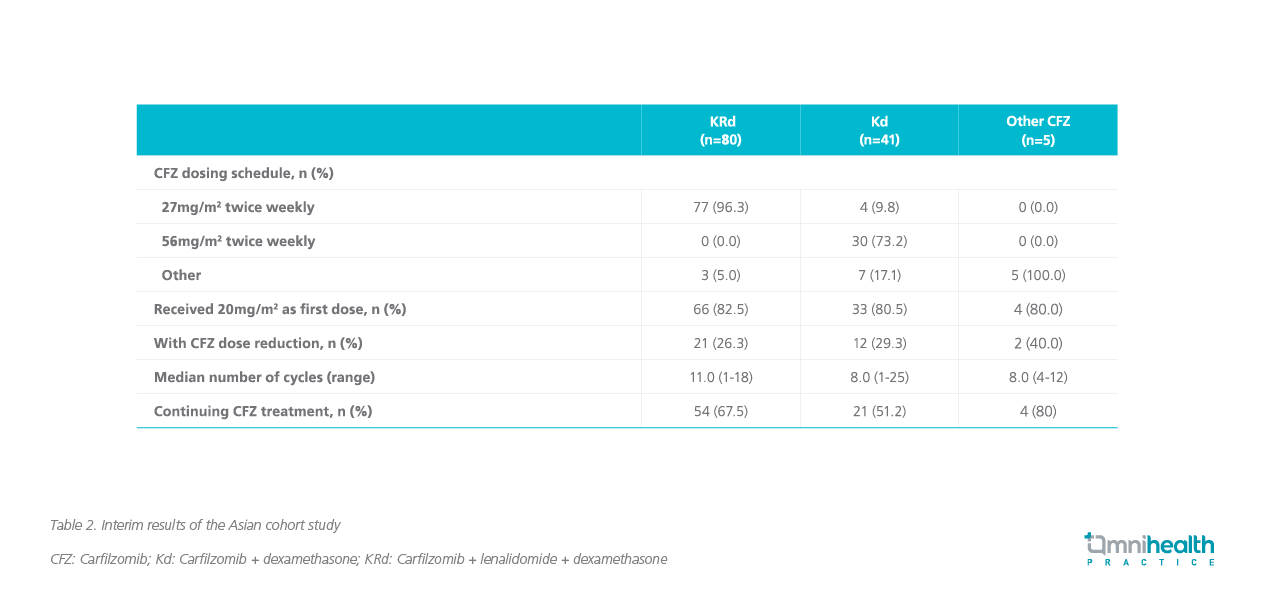
The interim results of this Asian cohort study (as of September 2020) found that more than 70% of patients in the KRd and Kd groups did not require dose modification, and the median cycles for KRd and Kd were 11 and 8, respectively (table 2).6 The majority of patients continued the carfilzomib-based rrMM treatments (KRd: 67.5%; Kd: 51.2%).6
Dr. Kong stated that the interim results of this Asian cohort study were consistent with the real-life clinical situation in Hong Kong. The median cycles of carfilzomib-based treatments in local patients were about 10, and most patients did not require dose reduction as carfilzomib was generally well tolerated. Regarding the final results of the study, Dr. Kong anticipated the forthcoming survival outcomes would be inferior to those demonstrated in the clinical trials. First, Dr. Kong stressed that there was an intrinsic difference in patient demographics between the RW study and the clinical trials, and therefore direct cross-study comparison may not be appropriate. In the RW setting, more patients had high cytogenic risk and were prone to have poor outcomes. In addition, due to the tremendous changes in rrMM treatment landscape over the past 5 years, he believed that the introduction of some highly potent drugs in the frontline treatment of rrMM would delay the use of carfilzomib-based regimen, thus shortening its PFS.
A local case sharing of applying the Kd regimen
Dr. Kong adopted the carfilzomib-based regimen in his practice and shared a case to demonstrate the effectiveness of Kd in the local clinical setting.
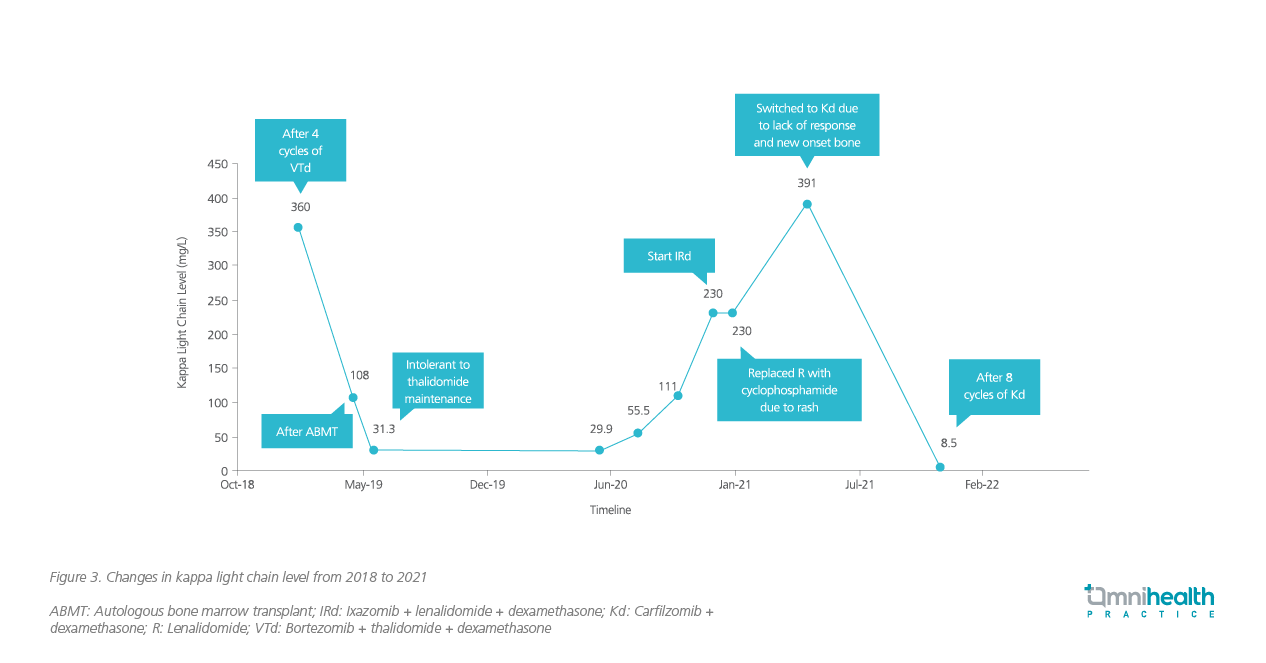
A lady in her 60s with no past medical history and cardiovascular (CV) risk factors was diagnosed with the International Staging System (ISS) stage 3 kappa light chain myeloma in 2018. She sought medical attention due to peripheral edema. The initial diagnostic workup identified acute renal injury, and the subsequent renal biopsy confirmed cast nephropathy. Bone marrow biopsy was also performed and found 95% of plasma cells in the bone marrow. However, cytogenetic risk by fluorescence in situ hybridization (FISH) test was not available due to sample problem.
The upfront MM treatment for this lady was 4 cycles of bortezomib + thalidomide + dexamethasone (VTd). Upon completion of 4 cycles of VTd, a very good partial response was achieved, and the initial serum Kappa light chain level was lowered from 17,302mg/L to 360mg/L (figure 3). Nevertheless, the patient could not tolerate immunomodulatory imide drugs (IMiDs) well and developed rash.
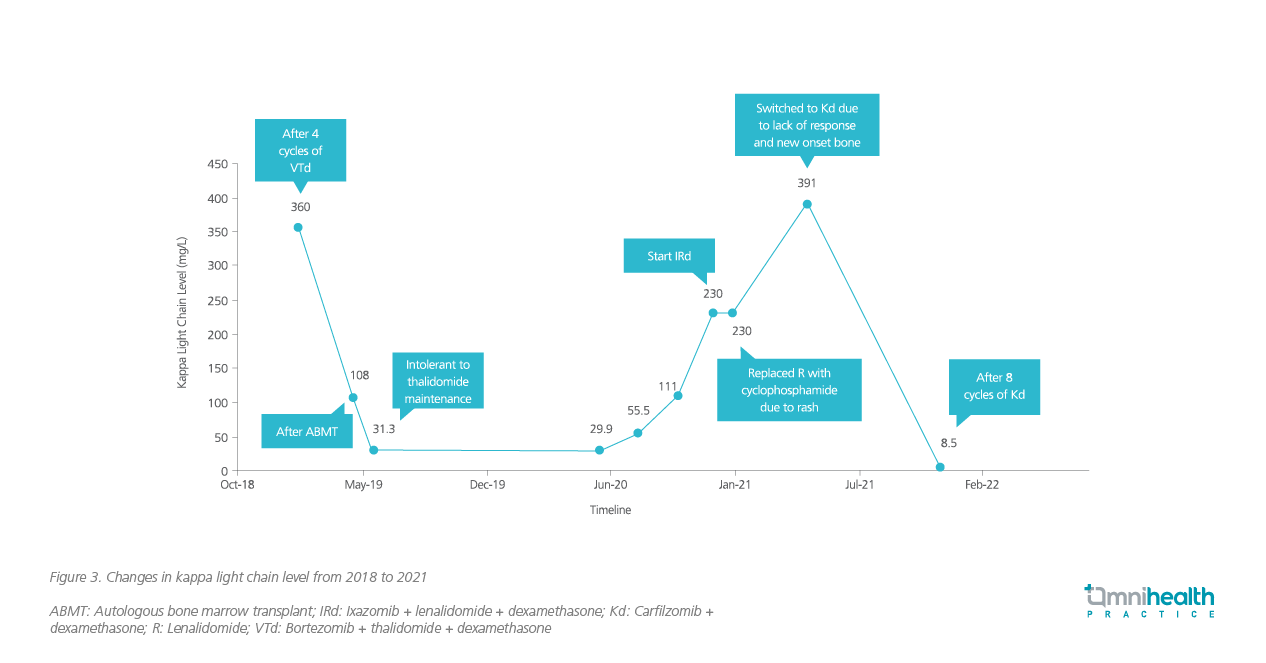
In May 2019, autologous bone marrow transplant (ABMT) was performed on the lady. After transplant, she was rechallenged with thalidomide as maintenance therapy. However, she still could not tolerate it. She was then given triplet therapy of ixazomib, lenalidomide and dexamethasone (IRd) in December 2020 for relapsed myeloma. Since the patient developed rash again, lenalidomide was replaced with cyclophosphamide, and 5 cycles of ixazomib + cyclophosphamide + dexamethasone (ICyD) were prescribed. The ICyD treatment was terminated due to the new-onset bone pain and the lack of improvement in reducing the serum kappa light chain level. The patient was given bisphosphonates for her bone pain and was switched to the duplet therapy of Kd.
After giving 8 cycles of Kd to the lady, the serum Kappa light chain level dropped from 391mg/L to the normal level of 8.5mg/L (figure 3). Since this patient was heavily pretreated, the signs of CV events were closely monitored. Throughout the treatment, she tolerated Kd treatment well and did not experience any notable adverse events (AEs). No CV events or fluid overload during administration were observed.
Dr. Kong concluded that rrMM patients often have a long treatment journey and require multiple lines of therapy to control their disease. Some treatment options, despite being effective, are associated with disturbing AEs which seriously affect patients’ quality of life. In this case, the patient switched treatments due to development of rash and bone pain, and eventually settled on the Kd regimen. The case showed that Kd was an effective and safe treatment option for rrMM patients, including those who have been heavily pretreated.
Conclusion
Overall, the interim data from the Asian study and the local experience showed that carfilzomib is an effective treatment option for controlling rrMM with high efficacy, manageable safety profile and convenient administration. Yet, whether or not carfilzomib therapies can achieve the equally remarkable survival benefits in the RW setting as in the clinical trials remains to be examined. More RW data on the carfilzomib-based regimen are needed to confirm its effectiveness in the Asian population with rrMM and help guide the local clinical practice.

