CONFERENCE UPDATE: WCLC 2021
Biomarker testing to aid in lung cancer diagnosis
Lung cancer management has been substantially changed, along with the recent advancements in cancer genomics and targeted therapies.1 A report released in 2020 by the American Association for Cancer Research (AACR) addressed disparities in managing cancer patients. Specifically, one section of which focused on the “imprecision of precision medicine” due partly to the limited understanding of the etiology and genetics of cancer within underserved racial and ethnic populations.1
Several factors may contribute to the disparities in biomarker testing, such as environmental factors, access to quality healthcare, insurance status, mistrust in the healthcare system, and the extent to which clinicians and patients understand the importance of biomarker testing under the treatment plan.1 Professor Leigh Boehmer, chief medical officer of the Association of Community Cancer Centres (ACCC), presented a study at the International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer, identifying the key areas of ongoing clinicians’ need related to biomarker testing. Results showed that less than half of the respondents in the community setting used biomarker testing to guide patient discussions about prognosis compared with nearly three-quarters of academic clinicians who did so.1 “Professional organizations and advocacy groups should focus on developing impactful education materials and tools for improving patient-clinician discussions about biomarker testing,” remarked Professor Leigh Boehmer.2 Besides, clinicians require more published guidelines and practical implications of clinical data, in addition to the information on financial resources for patient assistance.1
To address the unmet needs of free personalized, unlimited treatment navigation and clinical trial matching, Professor Daniel Saez, from GO2 Foundation for Lung Cancer, said the Foundation has developed a LungMATCH program that empowers the patients and caregivers in their treatment journey by providing access to the information regarding specific type of lung cancer.3 In addition to free educational materials, ranging from brochures to a quarterly educational newsletter, this program also provides information on biomarker test interpretation, as well as acquisition to interpretation of the medical science pertaining to the next line of treatment and nation-wide clinical trial searches.3 In a clinical trial from 2018 to 2020, data regarding history of all treatments were collected among patients who reported biomarker testing through phone or emails.3 To account for longitudinal consistency, mean values were used to transform and interpret the data. Clinical trial accrual rates were measured only for patients or caregivers who identified clinical trials as a potential treatment option.3 As measured by the targeted immunotherapy and biomarker testing rates, increased use of precision medicine among patients and clinicians was reported over the years. It was also found that clinical trial accrual rates increased each year and remained higher than the national average.3 (Figure 1)
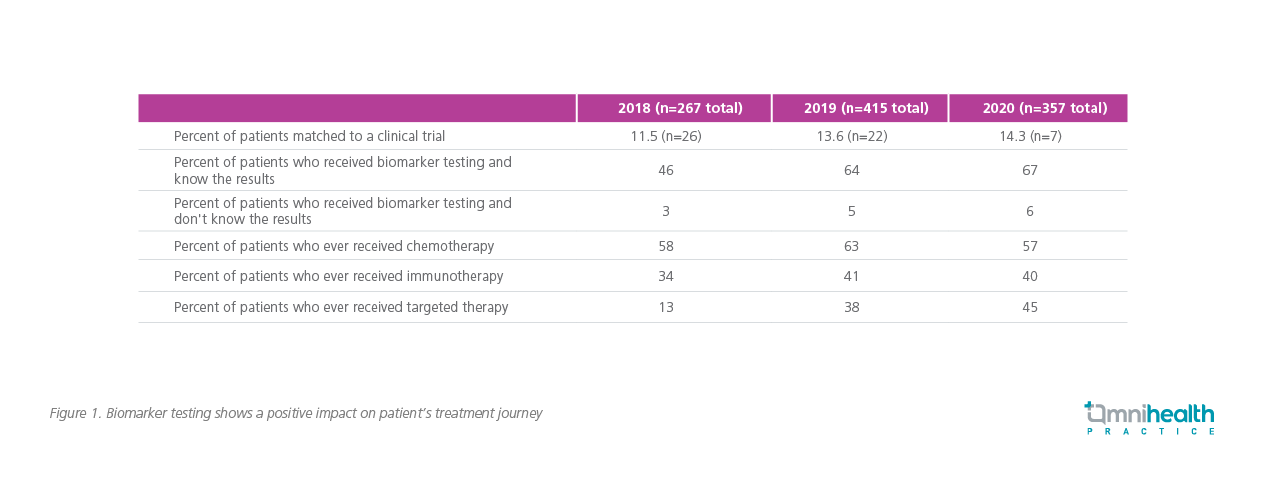
An interventional project is underway to pilot a lung cancer biomarker testing care sequence plan designed to promote patient engagement and offer a coordinated treatment approach.1 As stated by NCCN Clinical Practice Guidelines in Oncology, the analysis of quality of care will contribute to the future directions of the LungMATCH program, also pointing to the identification of correlative practices.4

