MEETING HIGHLIGHT
Defying the odds: Embarking on the new era of personalized immunotherapies for small cell lung cancer subtypes
In the Immuno-Oncology Hong Kong 2021, Dr. Lauren Averett Byers, Associate Professor from the Department of Thoracic/Head and Neck Medical Oncology of the University of Texas MD Anderson Cancer Center, United States, and Dr. Kristin Higgins, Associate Professor from the Department of Radiation Oncology of the Emory University School of Medicine and Medical Director of Radiation Oncology of the Emory Clinic, United States, discussed the identification of immunotherapy responders by small cell lung cancer subtyping and the role of prophylactic cranial irradiation (PCI) and thoracic radiation therapy (TRT) in the treatment of extensive stage-small cell lung cancer (ES-SCLC).
SCLC subtypes respond differently to immunotherapy
SCLC is an aggressive lung cancer characterized by rapid doubling time, early metastasis (approximate 60-70% of patients present with metastasis at the time of diagnosis), and poor overall prognosis.1 It is typically responsive to chemotherapy and radiation, while targeted therapies have not been approved for treatment.1 As such, there is an unmet need for personalized, biomarker-driven therapies for SCLC.
The molecular and clinical heterogeneity of SCLC was recently revealed by transcriptomic profiling and was classified into four SCLC subtypes, namely SCLC-A, -N, -P and -I subtypes.2 The oncogenesis of the SCLC-A, -N and -P subtypes was driven by the upregulation of distinct transcription factors namely ASCL-1, NEUROD-1 and POU2F3, respectively.2 On the other hand, SCLC-I represents a novel inflamed SCLC subtype with high expression of multiple immune-checkpoint genes but absence of dysregulation of the three known oncogenic transcription factors.2 Dr. Byers explained, “This inflamed group [SCLC-I] had not been previously seen or discovered in small cell lung cancer and could possibly be the group of patients that are getting relatively greater benefit from immune checkpoint blockades.”
Dr. Byers and her research team validated the four SCLC subtypes using 276 samples collected from ES-SCLC patients in the phase 3 IMP133 clinical trial.2 Through the retrospective analysis of IMP133 data, Dr. Byers and her team determined the efficacy of immune-checkpoint inhibitors (ICI) on the SCLC-I subtype.2 Transcriptomic profiling revealed that there were four distinct genomic signatures among these ES-SCLC specimens. Where the SCLC-A, -N, and -P subtypes were associated with high expression of ASCL1 (SCLC-A), NEUROD1 (SCLC-N) and POU2F3 (SCLC-P), the SCLC-I subtype was linked to high expression of immune-related genes, including IFNG, STING pathway, immune-checkpoints, and human leukocyte antigens (HLA).2
Consistent to the transcriptome data, SCLC-I patients formed a distinct epidemiological group with improved survival benefits following the treatment with etoposide-platinum (EP) plus atezolizumab, an immune checkpoint inhibitor (ICI), when compared with other SCLC-subtypes (HR=0.566; 95% CI: 0.321-0.998).2 In particular, the median overall survival (OS) of SCLC-I patients receiving EP plus atezolizumab vs. those receiving EP plus placebo were 18.2 months and 10.4 months, respectively.2 This difference may be attributed to the remarkable upregulation of HLA genes in the inflamed group (SCLC-I) when the majority of HLA genes were shut down in other SCLC-subtypes.2
Subsequently, these distinct genetic expression profiles between SCLC subtypes resulted in differential responses to treatment (Figure 1).2 During experimental exposure to different drugs, the SCLC-P subtype remained sensitive to poly-adenosine diphosphate-ribose polymerase (PARP) inhibitors as well as aurora kinase inhibitors whereas the SCLC-I type became highly resistant to PARP inhibitors.2,3 These indicated that the inflamed subtype SCLC-I may acquire drug resistance during treatment with PARP inhibitors.3 However, SCLC-I remained susceptible to immunotherapy.3
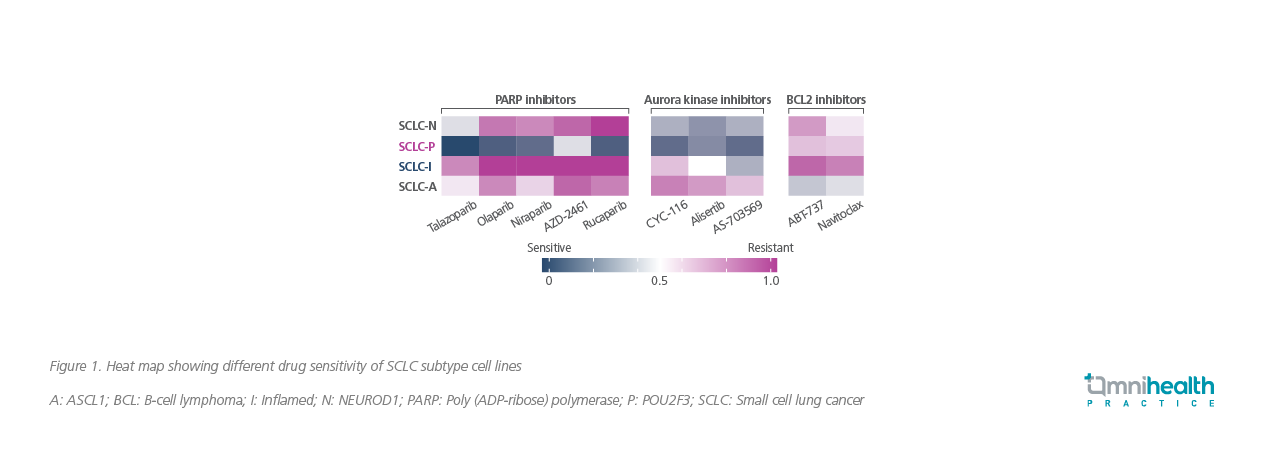
Collectively, molecular subtyping of SCLC can potentially open up a new avenue for development of personalized therapy for SCLC patients.2 However, it should be noted that SCLC tumors are highly heterogeneous by the time of diagnosis and may encompass more than one subtype.2 Moreover, the tumor cells can quickly adapt to a given drug and acquire drug resistance by subtype-switching.4-6 Dr. Byers explained that following treatment with cisplatin with other targeted therapies, there would be a subset of patients whose tumors are switching over to a more inflamed phenotype, highlighting, “Switching between subtypes may be an important mechanism of resistance and figuring out how to block that switching or switch tumors back may be important in terms of a therapeutic approach to resistance.”
The CASPIAN and IMP133 trials support the addition of durvalumab or atezolizumab to standard chemotherapy for ES-SCLC
Two landmark studies, CASPIAN and IMP133, have opened up a new therapeutic standard of adding immunotherapy to standard platinum-based chemotherapy for the treatment of ES-SCLC.7,8 To date, durvalumab and atezolizumab have been approved by the United States Food and Drug Administration Agency (FDA) based on the positive findings from the CASPIAN and IMP133 trials.9,10
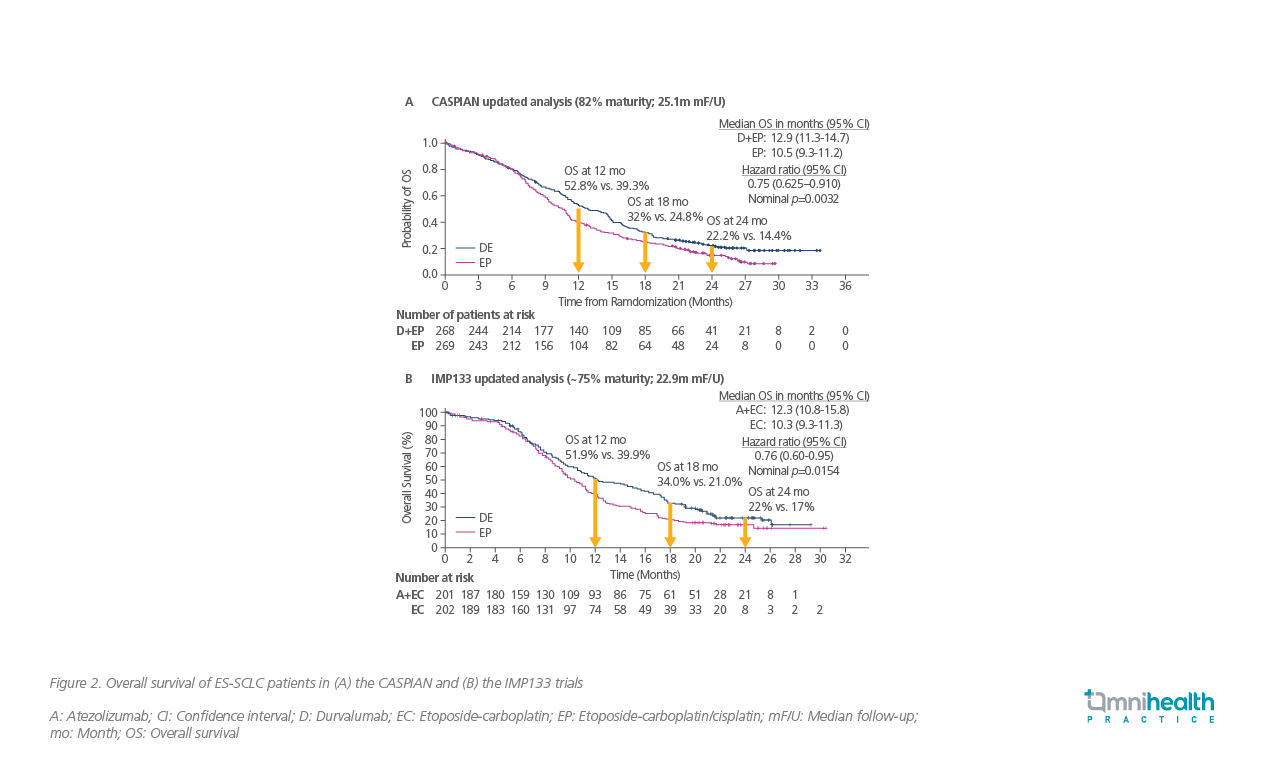
The CASPIAN study was a randomized, open-label, phase 3 clinical trial involving untreated ES-SCLC patients from 23 countries.7 At 24 months, 22.2% of patients receiving durvalumab plus EP survived as compared with 14.4% in the placebo group receiving EP alone (HR=0.75; 95% CI: 0.625-0.910; p=0.0032) (Figure 2A).7
A similar survival benefit was also observed in the IMP133 trial which evaluated the OS benefit of atezolizumab plus etoposide-carboplatin (EC) vs. EC alone in patients with SCLC.8 At 24 months, 22% of patients in the atezolizumab arm vs. 17% of patients in the placebo arm were alive (HR=0.76; 95% CI: 0.60-0.95; p=0.0154) (Figure 2B).8,11
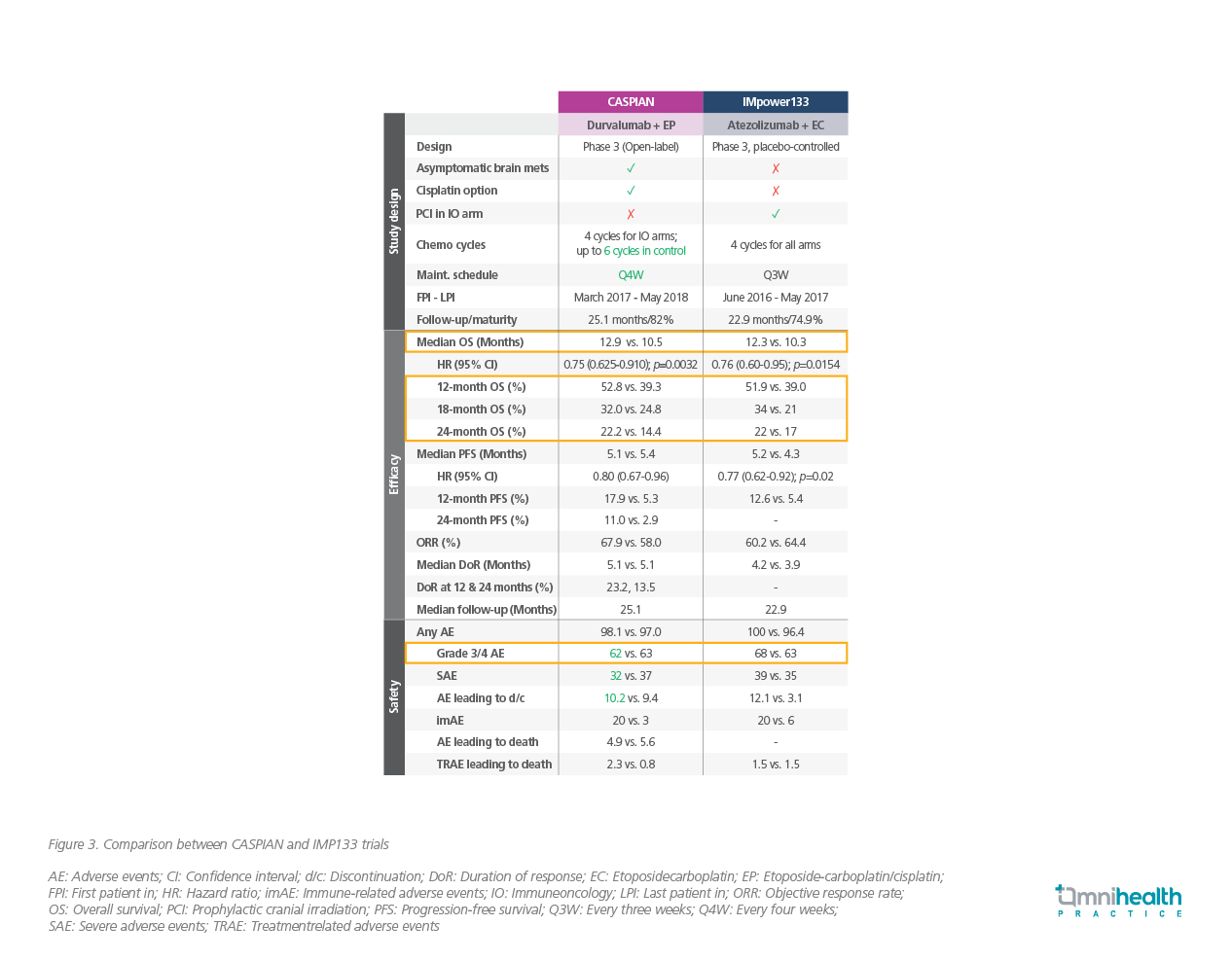
While both the CASPIAN and IMP133 trials confirmed the efficacy of ICIs in combination of standard platinum-based chemotherapy, there were a number of differences between two studies (Figure 3).7,8,11 In the IMP133 trial, patients with treated asymptomatic brain metastases were eligible (those with active or untreated CNS metastases were excluded from the study) and treatment arms did not include the use of cisplatin (only carboplatin was used) with etoposde.8,11 In the CASPIAN trial, patients with brain metastases were eligible provided that they were either asymptomatic or treated and stable off steroids and anticonvulsants for at least 1 month before study entry and there was cisplatin option available to be given with etoposide.7 Although the inclusion criteria of the study population were different, both durvalumab and atezolizumab in combination with platinum-based chemotherapy conferred better survival in similar extent, with a low risk of additional adverse events (AEs).7,8,11
Ongoing studies to improve therapeutic outcomes in SCLC patients
Based on the discovery of the four SCLC subtypes, including the novel inflamed SCLC-I subtype which has a preferential benefit in immunotherapy, there are several clinical trials underway to develop a clinical subtype biomarker strategy for use in patients. Dr. Byers highlighted an ongoing phase 1/2 clinical trials investigating the combination of olaparib, durvalumab plus TRT as a consolidation therapy after chemotherapy and durvalumab (NCT04728230).12 Another trial which aims to evaluate AZD2811, an aurora kinase inhibitor, in combination with durvalumab as maintenance therapy after induction with platinum-based chemotherapy combined with durvalumab (NCT04745689) is also under study.13 The results of these trials are expected to be available in 2024.
Leveraging RT and immunotherapy as a synergistic approach towards SCLC treatment
In addition to immunotherapy and platinum-based chemotherapy, radiotherapy (RT) is also a cornerstone of treatment for SCLC.14 Previously, the benefit of adding TRT to platinum-based chemotherapy was well-demonstrated in randomized controlled trials in prolonging patient survival and symptom palliation in ES-SCLC.14 However, the role of PCI in patients with ES-SCLC after treatment with EP remains controversial, with conflicting evidence regarding its potential survival benefit.15
As shown in a Japanese phase 3, open-label, multicenter, randomized trial, PCI (n=113) did not result in a longer OS compared with observation (n=111) in patients with ES-SCLC.16 Of note, anorexia, nausea, and malaise, were the most frequently observed grade 3 or worse adverse events at 3 months.16 The study concluded that PCI is not essential for patients with ES-SCLC with any response to initial chemotherapy and a confirmed absence of brain metastases when patients received periodic MRI examination during follow-up.16 Additionally, if the cost of the observation strategy (magnetic resonance imaging [MRI] plus radiotherapy for brain metastases) is lower than that of PCI (PCI plus MRI plus radiotherapy for brain metastases), the observation strategy could be recommended because of the equal survival.16
This Japanese study appears to be the driving force for the reduction of PCI use for ES-SCLC, reported by a survey involving 3,851 radiation oncologists in the United States of America.17 In the survey, while 72% of them reported that they routinely offered PCI to ES-SCLC patients prior to the publication of this Japanese trial, only 44% continue to do so currently (difference=28%; 95% CI: 25%-31%; p<0.001).17 Of the respondents not aware of this Japanese study, 85% continued to offer PCI, a higher percentage than those who were aware of this study (odds ratio=0.11; 95% CI: 0.04-0.32; p<0.001).17 These results highlighted the continued lack of consensus for PCI in SCLC and supported ongoing investigations, such as MAVERICK (SWOG-1827).17
When considering add-on treatment to RT, immunotherapy was also shown to have potential synergy in treating SCLC. Mechanistically, the potential synergy between immunotherapy and RT has been built on the immunomodulatory effect of RT through priming dendritic cells, increasing tumor antigen release, upregulating immunogenic cell surface markers which eventually enhance T-cell mediated tumor cell specific killing.18 To further support this potential synergy, Dr. Higgins presented unpublished data based on re-analysis of the IMP133 cohort. The median time to intra-cranial progression for patients receiving platinum-based chemotherapy plus atezolizumab vs. patients receiving platinum-based chemotherapy plus placebo were 20.2 months and 10.5 months (HR=0.66; 95% CI: 0.44-1.00; p=0.046), respectively.
Thoracic radiation is currently being studied in the setting of immunotherapy in SCLC; multiple clinical trials are underway to test optimal use of radiotherapy in combination with immunotherapy including RAPTOR, and NRG LU005, trials.19-26 These trials evaluated durvalumab and other ICIs in the setting of chemoradiation therapy and the results are expected to be available in a few years.21-24 In conclusion, Dr. Higgins said, “There are certainly new therapeutic options finally here for SCLC. We are hopeful that radiation and immunotherapy can synergize.”
Durvalumab is efficacious for SCLC-I subtype and currently being tested as combination therapy with RT
In the panel discussion, Dr. Byers highlighted that the efficacy and safety of durvalumab have been proven in ES-SCLC patients with asymptomatic brain metastases in the CASPIAN trial, encouraging an early initiation of treatment when diagnosing brain metastasis during staging. As it is challenging to prescribe whole-brain radiation therapy (WBRT) in 30Gy and reduce patients’ performance status before systemic treatment, the results in CASPIAN trial are therefore helpful for the development of treatment strategies in SCLC, noted Dr. Higgins.
When asked about the preference of choosing between durvalumab and atezolizumab, Dr. Byers and Dr. Higgins opined that it would be institution dependent and with both agents being equally effective. Dr. Higgins emphasized that there are currently multiple clinical trials testing the combination of durvalumab and RT. She believes durvalumab could work synergistically with RT. In light of the discovery of the SCLC-I subtype which could be resistant to DNA damaging therapies such as RT, immunotherapy may play an important role in SCLC treatment. Dr. Higgins suggested that RT can be administered in between immunotherapy maintenance cycles to maximize the treatment benefit.
Conclusion
In summary, as immunotherapy adding to standard chemotherapy gives rise to survival benefits in SCLC patients, they may become the new potential standard-of-care with personalized treatment strategy for each SCLC subtype. Although PCI plays a controversial role in ES-SCLC, the synergy between immunotherapy and thoracic radiation should be leveraged as an approach to improve outcomes in SCLC.

