CASE REVIEW
Bridging towards allogeneic stem-cell transplant with the early use of novel therapies in the relapsed/refractory acute lymphoblastic leukemia setting
Complete remission (CR) is a prerequisite to allogenic stem-cell transplant (allo-SCT) which remains to be the only curative option in adult with relapsed/refractory acute lymphoblastic leukemia (R/R ALL).1 However, conventional cytotoxic chemotherapies can only achieve CR in 40-50% of adult ALL patients which subsequently reduce the number of patients eligible for allo-SCT.2 In a recent interview with Omnihealth Practice, Professor Tse, Wai-Choi Eric, Clinical Professor at the Department of Medicine, Li Ka Shing Faculty of Medicine, the University of Hong Kong, shared a patient case where novel therapies can help achieve CR and recommended the earlier use of novel therapies to bridge towards allo-SCT.
Background
ALL is an aggressive and highly fatal malignancy that occurs predominantly in children and adults around the age of 50-years old. 3 Where the conventional cytotoxic chemotherapies can achieve a 90% and 60% cure rate in children and adolescents with newly diagnosed ALL, respectively, 40-50% of adult ALL patients will experience disease relapse.2 Other than adult ALL patients, those diagnosed with Philadelphia chromosome (Ph)- positive ALL are also known to have poorer prognosis.4 For these patients, the incorporation of imatinib, a tyrosine kinase inhibitor (TKI), into standard chemotherapies can significantly improve the CR rate to 92% versus 82% with chemotherapy alone (p=0.004).4 However, Ph-positive patients treated with imatinib plus chemotherapy only have a 50% 4-year relapse-free survival rate and would eventually require allo-SCT, the only curative option for adult R/R ALL.1
In the local public hospital setting, R/R ALL patients are typically treated with coventional chemotherapy as bridging therapy to transplant if a suitable donor is available and that the patient is fit for transplant. However, conventional cytotoxic chemotherapies in the R/R ALL setting are associated with poor CR rates of only 40% in first salvage that deteriorates to 21% after the second salvage and 11% of third or greater salvage.1 From clinical experience, older patients typically cannot tolerate intense chemotherapy which results in an even higher relapse rate of 70-80%. Although more Ph-positive ALL patients can achieve allo-SCT (46% vs. 31%) and event-free survival (HR=0.64; 95% CI: 0.44-0.93; p=0.02) with the addition of imatinib, treatment options remain limited in the R/R setting to achieve CR for allo-SCT initiation and necessitates the development of novel therapies for outcome improvement.4
Available novel treatment options to help achieve allo-SCT in the R/R setting
To help achieve CR and enable allo-SCT, inotuzumab ozogamicin (InO), a humanized anti-CD22 monoclonal antibody conjugated to calicheamicin (a cytotoxic antibiotic agent), is one such novel therapy having this potential.5 In the phase 3 INO-VATE study (n=326), R/R ALL patients who received InO had significantly improved CR or CR with incomplete hematologic recovery (CRi; 73.8% vs. 30.9%; pone-sided<0.0001) and demonstrated a longer duration of remission (median=5.4 vs. 4.2 months; HR=0.62; 95% CI: 0.42-0.91; pone-sided=0.0071) when compared to standard intensive chemotherapy.5 Notably, 2.8 times more patients receiving InO achieved minimal residual disease (MRD) – negative with a threshold of 0.01% marrow blasts (78.4% vs. 28.1%; p<0.001), a key prognostic indicator for improved overall survival (OS) and progression-free survival (PFS) when paired with CR/CRi than MRD-positive patients.6,7
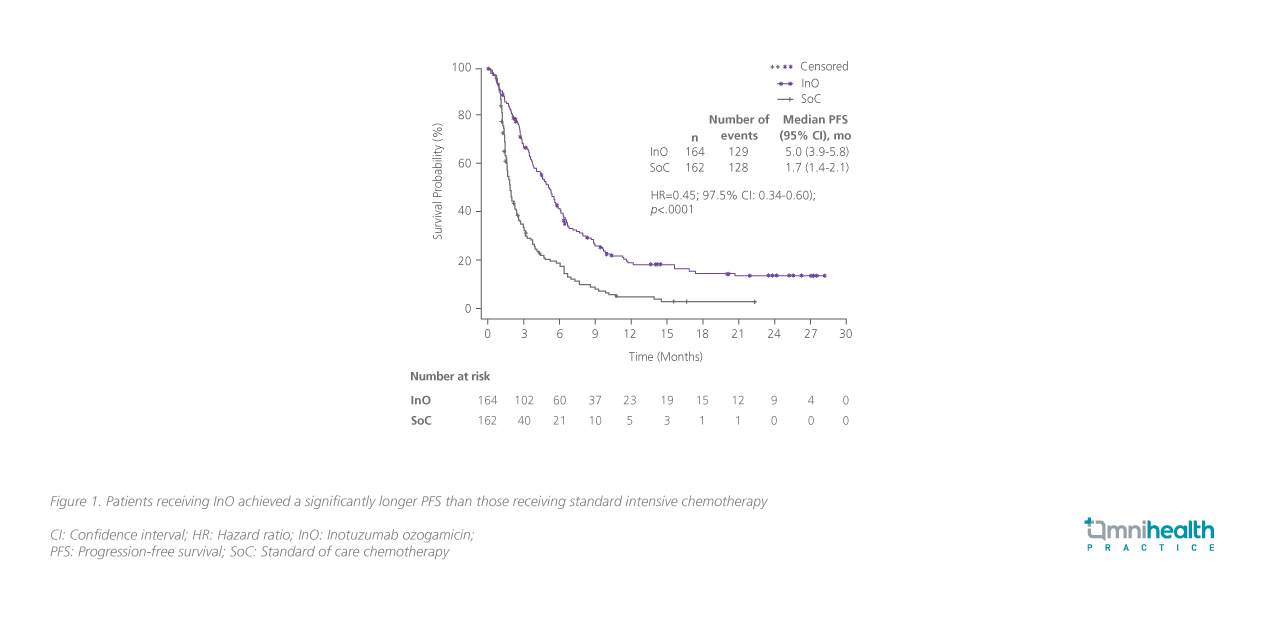
In the survival analysis, InO significantly improved median PFS (5.0 vs. 1.7 months; HR=0.45; pone-sided<0.0001; Figure 1) and median OS (7.7 vs. 6.2 months; HR=0.75; pone-sided=0.0105) with the survival benefit remaining consistent regardless of the presence of Ph-positive or t(4;11) mutations.5 With improved CR/CRi and MRD-negative rates, nearly 4 times more patients proceeded directly to allo-SCT after InO (39.6% vs. 10.5%; pone-sided<0.0001).5 Even after allo-SCT, InO continued to demonstrate a trend in survival benefit with a lower cumulative incidence rate of relapse (28.6% vs. 45.7%; subdistribution HR=0.65; 97.5% CI: 0.34-1.24; p=0.18) and improved OS (41.4% vs. 34.1%; subdistribution HR=1.26; 97.5% CI: 0.66-2.41; p=0.78) at 24 months post-transplant when compared to standard chemotherapy.8
Blinatumomab, a bispecific T-cell engager (BiTE), is another novel therapy that is available for adult R/R ALL patients.9 In the phase 3 TOWER study (n=405), blinatumomab improved median OS (7.7 vs. 4.0 months; HR=0.71; 95% CI: 0.55-0.93; p=0.01) with significantly more patients achieving CR (34% vs. 16%; p<0.001) and CRi (44% vs. 25%; p<0.001) than standard chemotherapy.9 Although blinatumomab did not increase the number of patients undergoing allo-SCT when compared to chemotherapy alone (both 24%), blinatumomab improved the rates of CR/CRi and thus increased the number of patients eligible for allo-SCT.9 In practice, InO should be considered after failing blinatumomab and vice versa as both novel agents can improve CR/CRi while having a different target and mechanism of action. Importantly, both InO and blinatumomab only achieved a duration of remission of <1 year, highlighting the value to reach CR/CRi as soon as possible to initiate allo-SCT when the R/R ALL patient is still in remission. To illustrate the use of novel therapies in the R/R ALL setting, a local patient case was shared.
Case Report
A 65-year-old lady was presented to the clinic with newly diagnosed ALL. After genomic screening, she was further diagnosed with Ph-positive ALL and the standard of care of conventional chemotherapy plus imatinib was given as first-line treatment.
Although a brief clinical remission was achieved, she experienced disease relapse shortly during treatment and imatinib was switched to the second-generation TKI dasatinib. As her disease was not controlled after a few cycles of treatment, another genomic investigation was performed and revealed that she had a T315I BCL-ABL molecular mutation, suggesting that she was resistant to most TKIs but ponatinib. The TKI was then switched to ponatinib, but her disease remained uncontrolled. Under compassionate use, she initiated blinatumomab but failed to achieve CR/CRi. She subsequently received InO with goal to achieve better disease control.
Impressively, she achieved CR after 1 cycle of InO despite already receiving multiple lines of prior therapies. However, her platelet count did not recover despite having no obvious increase in bone marrow blasts. While routine platelet transfusion was still required, she reported improved quality-of-life with less red cell transfusion and chemotherapy required to manage her disease. Overall, InO was well-tolerated and she only experienced transient liver function derangement.
Unfortunately, she had severe infection before receiving the third cycle of InO. Subsequently, her ALL had an explosive progression and she passed away shortly after.
Discussion
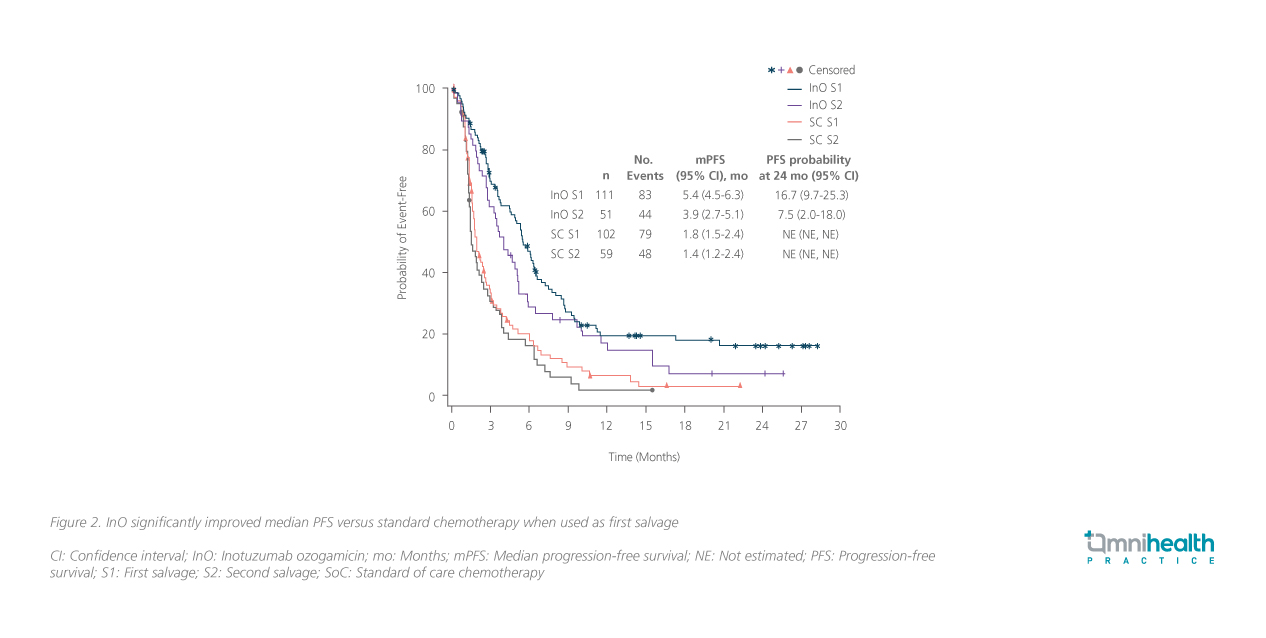
Although the R/R ALL patient in the shared case was not suitable for allo-SCT due to her old age, InO was able to maintain control of her disease for 2 months even after numerous lines of prior treatments. Where InO was given in the later lines of treatment in the shared case, based on the in-depth analysis of the INOVATE study, patients receiving InO achieved higher rates of CR/CRi than standard chemotherapy regardless of salvage stage but the benefit was more pronounced in first savage (Salvage 1: 78.4% vs. 28.4%; p<0.0001; Salvage 2: 64.7% vs. 35.6%; p=0.0012).10 When considering survival benefit, patients receiving InO as first salvage also derived greater benefit in median PFS (5.4 vs. 1.8 months; HR=0.44; 97.5% CI: 0.31-0.64; p<0.0001) than second salvage (3.9 vs. 1.4 months; HR=0.49; 97.5% CI: 0.30-0.81; p=0.0006) when compared to chemotherapy, supporting the use of novel therapies in earlier lines of R/R ALL treatment to bridge towards allo-SCT (Figure 2).10
Importantly, InO also improved the rates of CR/CRi and OS regardless of underlying disease burden.11 Among patients with low, moderate or high disease burden as defined by BMB <50%, 50-90% and >90%, InO consistently improved CR/CRi (74% vs. 46%; p=0.0022, 75% vs. 27%; p<0.0001 and 70% vs. 17%; p<0.0001, respectively) and OS (HR=0.64; p=0.0260, 0.81; p=0.1109 and 0.60; p=0.0335, respectively) when compared to standard intensive.11 As such, the selection of novel agents such as InO should not be limited by previous therapy choices and should be considered as early as possible for all R/R ALL patients.
Other than CR/CRi, MRD status is also a key prognostic indicator of outcome among R/R ALL patients and should be a treatment goal when treating R/R ALL.7 Compared to MRD-positive ALL patients, those who achieved MRD-negative and CR/CRi with InO were associated with significantly improved median PFS (8.6 vs. 5.4 months; HR=0.423; 97.5% CI: 0.256-0.699; pone-sided<0.0001) and median OS (14.1 vs. 7.2 months; HR=0.512; 97.5% CI: 0.313-0.835; pone-sided<0.0009).7 Notably, more MRD-negative patients proceeded to allo-SCT after InO as both first (66% vs. 50%) and second salvage (63 vs. 41%) than MRD-positive patients.7 Even among patients who did not receive follow-up allo-SCT, those who achieved MRD-negative with InO had longer median OS than those who were MRD-positive (9.4 vs. 6.3 months; HR=0.47; 97.5% CI: 0.23-0.98; pone-sided=0.0088).7 When combined with allo-SCT, MRD-negative and CR/CRi R/R ALL patients can achieve the best survival outcome with InO with a median OS of 21.5 months and median PFS of 8.6 months.7 Although routine MRD assessment was only established in this recent year in local practice, near 70% of patients can achieve MRD-negative and CR/CRi with InO which is substantially higher than those receiving chemotherapy, supporting the early use of novel therapies for all R/R ALL patients to maximize their survival benefits even if the patients are not suitable for allo-SCT.7
In terms of safety, while InO was well-tolerated by the patient in the shared case as she only experienced transient liver function derangement, InO is associated with an increased risk of Veno-occlusive disease (VOD) and multiple cycles of treatment should be avoided.5 As VOD can also be induced by high doses of chemotherapy or radiation therapy prior to allo-SCT, it is ideal to achieve CR/CRi within 2-3 cycles of InO to avoid further hepatic complications. When needed, ursodiol can be prescribed as prophylaxis for patients at risk of VOD. Otherwise, patients with reduced liver function such as those receiving prior L-asparaginase should delay InO until liver function recovers.
Conclusion
For adult R/R ALL, older adults with ALL tend to have higher risk factors at diagnosis with more comorbidities that require dose reductions during induction chemotherapy, potentially reducing the rates of CR/CRi and thus the eligibility towards allo-SCT.2 Encouragingly, novel therapies including InO and blinatumomab can achieve high rates of CR/CRi and MRD-negative in adult R/R ALL, serving as reliable bridging tools to allo-SCT. With data supporting the earlier use of novel therapies benefiting patients with R/R ALL, it is recommended to use these novel agents early to achieve CR/CRi and bridge towards allo-SCT.

