EXPERT INSIGHT
Improving the management of advanced and late-line gists with dual action tyrosine kinase inhibitors
Gastrointestinal stromal tumors (GISTs) are the most common primary mesenchymal tumors of the gastrointestinal (GI) tract with an estimated incidence and prevalence of 14.5 and 129 per million population, respectively, in the United States.1 While imatinib is the standard treatment for locally advanced inoperable and metastatic disease, many GISTs will develop secondary mutations and increase the challenge for tyrosine kinase inhibition.2 In the recent interview with Omnihealth Practice, Dr. Margret von Mehren, Chief of the Division of Sarcoma Medical Oncology of the Fox Chase Cancer Center, United States, described the treatment approach towards patients with progressing GISTs. When considering treatment for patients with multiclonal disease, ripretinib, a next generation tyrosine kinase inhibitor (TKI), is hypothesized to be active against many types of secondary mutations and is recommended for patients who failed imatinib, sunitinib and regorafenib.3
Treating GISTs in the first-line setting
According to the joint clinical practice guidelines from the European Society for Medical Oncology and The European Reference Network on Rare Adult Solid Cancers (ESMO-EURACAN), the prognosis of GISTs is dependent on the tumor’s location, size, mitotic rate, and presence of rupture.2 Where the best prognosis is observed in patients with small, gastric GISTs with low mitotic count and no rupture, the worst prognosis is observed in those with large small-bowel or rectal GISTs with high mitotic count and tumor rupture.2
Although mutational status has not been incorporated in the risk classification of GISTs, their genetic features such as KIT and PDGFRA mutation status can help predict the response of advanced or metastatic GISTs towards TKI treatment.2,4 Where KIT mutations are typically associated with an increased risk of recurrence, KIT exon 9 mutants often require increased doses of imatinib for disease control.4 While GISTs with PDGFRA mutations are more indolent in nature and are generally associated with good prognosis, localized GISTs with PDGFRA D842V mutation at exon 18 can confer resistance towards imatinib.2 In practice, patients with KIT/PDGFRA wildtype and succinate dehydrogenase (SDH) deficient GISTs tend to have better prognosis even if the tumors cannot be surgically resected or are in the early metastatic stage. However, these patients are usually at an increased risk of pheochromocytoma and paraganglioma syndromes and should be germ-line tested to confirm SDH gene mutations. Otherwise, SDH-deficient and NF-1 related KIT/PDGFRA/BRAF wildtype tumors should avoid adjuvant treatment as these GISTs lack sensitivity towards imatinib and should consider resection where available.2
Management strategies for GISTs after failing imatinib
Even though most GISTs initially respond well to imatinib and can achieve clinically meaningful treatment results, almost all patients with GISTs will eventually acquire secondary mutations and become resistant to treatment.4 Indeed, while patients with advanced GISTs can achieve a median overall survival (OS) of 57 months with first-line imatinib, many will have disease progression within 2 years with a median time to progression of 24 months.5 In fact, 66% of treatment responding patients and 78% of patients with stable disease have disease progression at 60 months.5
At disease progression on imatinib, many GISTs acquire secondary mutations at the ATP-biding pocket (encoded by exons 13 and 14) and the activation loop (encoded by exons 17 and 18) of the kinase domain, resulting in progressive treatment resistance towards KIT- or PDGFRA-targeting TKIs.6 In the second-line setting, sunitinib, which primarily inhibits imatinib-resistance mutations in the ATP-binding pocket on exon 13 and 14, can significantly improve median progression-free survival (PFS) to 6.3 months versus 1.5 months in those receiving placebo (HR=0.33; p<0.0001).6,7 In GISTs that progressed on imatinib and sunitinib, treatment with regorafenib, which primarily inhibits imatinib-resistance mutations in the activation loop on exon 17, improved the median PFS of 4.8 months versus 0.9 months when compared to placebo (HR=0.27; 95% CI: 0.19-0.39; p<0.0001).6,8
While sunitinib and regorafenib have complementary activity against imatinib-resistance mutations, neither treatment covers the full spectrum of possible mutations in GISTs.3 Particularly, wildtype GISTs that are SDH deficient may benefit from the anti-vascular endothelial growth factor (VEGF) targeting of sunitinib and regorafenib, but NF-1 related wildtype GISTs may not respond to either treatment.2 Moreover, GISTs that fail imatinib, sunitinib and regorafenib usually become multiclonal with different secondary mutations across the metastatic GISTs in the body. Although avapritinib is available, the treatment is only approved for patients with PDGFRA mutations at exon 18 and its applicability may be limited in practice.3 As such, a treatment that can provide broad inhibition of KIT and PDGFRA kinase activity, including KIT/PDGFRA wildtypes and GISTs with multiple primary and secondary mutations, is needed for patients who have failed multiple TKI treatments.
The pan-KIT and pan-PDGFR benefit of ripretinib addresses drug resistance in GISTs
Ripretinib is a switch-control TKI with a dual mechanism of actions which broadly inhibits KIT and PDGFRA kinase signaling.3 By specifically and durably binding to both the switch pocket and the activation loop, ripretinib can lock the kinase in the inactive state and prevent downstream signaling and cell proliferation of GISTs.3 In vitro, ripretinib’s high affinity binding to KIT and PDGFRA receptors enabled potent antineoplastic effects KIT mutations in exons 9, 11, 13, 14, 17, and 18, and PDGFR mutations in exons 12, 14 and 18.9 As ripretinib does not bind to the ATP-binding pocket where most secondary mutations are acquired, it is hypothesized that ripretinib is active against many types of mutations, particularly in GISTs that have acquired multiple secondary mutations as disease progresses. With anti-VEGF targeting, ripretinib is also active against wildtype GISTs that are SDH deficient.
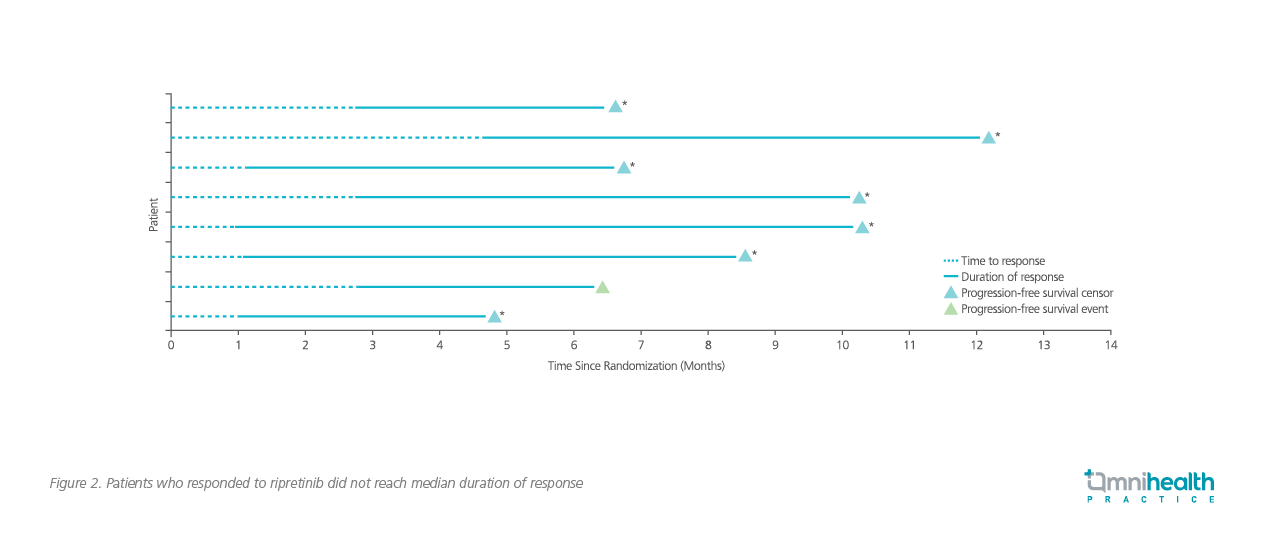
In the double-blind, randomized, placebo-controlled, phase 3 INVICTUS study (n=129), patients with advanced GISTs and progression on imatinib, sunitinib and regorafenib treatment were randomized 2:1 to receive either ripretinib or matching placebo.3 Ripretinib significantly improved median PFS to 6.3 months versus 1.0 months in patients receiving placebo (HR=0.15; 95% CI: 0.09-0.25; p<0.0001; Figure 1).3 Notably, 51% of patients on ripretinib achieved 6-month PFS which was dramatically higher than the 3.2% in those receiving placebo.3 In terms of OS, ripretinib achieved a significantly longer median OS of 15.1 months versus 6.6 months with placebo (HR=0.36; 95% CI: 0.21-0.62).3 Where 9.4% (8/85) of patients on ripretinib achieved a confirmed objective response (OR), none achieved OR on placebo (0/44).3 Remarkably, only 1 out of the 8 responders had disease progression at the time of data cut-off (Figure 2).3 While the study included an unselected population of patients with advanced GISTs including those with KIT/PDGFRA wildtype tumors, a consistent benefit in PFS improvement was observed across the study population, supporting the pan-KIT and pan-PDGFRA benefit of ripretinib that extends to tumors with wildtype KIT and PDGFRA receptors.3

In terms of safety, the most common treatment-related adverse events (≥20%) in patients receiving ripretinib were alopecia, myalgia, nausea, fatigue, hand-foot syndrome, and diarrhea.3 A majority of these adverse events were of grade 1-2 and only 6% and 5% of patients receiving ripretinib required dose reduction or discontinuation, respectively.3
Applying ripretinib in practice
As GISTs continue to progress after multiple lines of treatment, many tumors will acquire different patterns of resistance and become difficult to treat. At this stage, tumor shrinkage is rarely observed even when adequate treatment is given. As such, the treatment goal for patients failing imatinib, sunitinib and regorafenib should be targeted towards stable disease which can significantly contribute to long-term survival benefit. In the INVICTUS study, 66% of patients achieved stable disease at 6 weeks with ripretinib versus 21% of patients receiving placebo.3 Notably, this benefit was maintained at 12 weeks with 47% of patients on ripretinib remaining in stable disease versus 5% in those receiving placebo, supporting the benefit of ripretinib in patients who failed multiple lines of treatment.3
When treating GISTs in practice, the treatment sequence after acquiring imatinib resistance may be swapped based on the treatment sensitivity of GISTs. According to mutation testing, regorafenib may be considered before sunitinib if secondary mutations are not found on exon 13 or 14. However, a tumor biopsy can only represents a minor portion of a patient’s GIST and may not be representative of the genetic profile of the whole disease. In this regard, the pan-KIT and pan-PDGFR benefits of ripretinib can enable wide tumor control including multiclonal GISTs that cannot be targeted by sunitinib or regorafenib. Given the unique benefit of ripretinib in its dual mechanism of actions, a multicenter, open-label, randomized phase 3 INTRIGUE study is being conducted to investigate the efficacy of ripretinib versus sunitinib in patients with advanced GIST following imatinib treatment.9 With an estimated study completion date in March 2022, another treatment option may be available in the near future for patients with difficult-to-treat GISTs that have extensive KIT and PDGFRA mutations.9
When prescribing ripretinib for patients with GISTs, clinicians should monitor the patient’s blood count to identify rare cases of anemia, leukopenia and thrombocytopenia. As ripretinib targets VEGF, a mild elevation in blood pressure may be observed and should be continuously monitored. Although alopecia is a common concern among patients with GISTs receiving ripretinib, the hair thinning is often mild and the hair will typically grow back being coarser and curlier. As alopecia cannot be prevented, clinicians should educate patients on the possibility of hair loss before treatment to improve patient awareness. Myalgia is a common side effect of TKIs and can be circumvented by routine hydration and electrolytes intake. In the rare case of intolerable myalgia, the dose of ripretinib can be reduced and stepwise increased back to the target dose after the symptoms have subsided.
When patient progresses on ripretinib, clinicians should investigate on the site of progressions of GISTs. If the disease is multi-focal, another TKI can be adopted for better disease control. Alternatively, increasing the dose of ripretinib to 150mg twice daily may be considered as a well-tolerated regimen without clinically meaningful dose-limiting side effects.10 For GISTs that progress with only 1 to 2 metastatic sites, clinicians should consult surgeons and interventional radiologists to evaluate whether local therapies are available while continuing ripretinib.
Conclusion
As disease progresses, GISTs continue to acquire secondary mutations and become difficult to target with KIT and PDGFR-targeting TKIs. When patients fail imatinib, sunitinib and regorafenib, ripretinib, a switch-control TKI with broad inhibition of KIT and PDGFRA kinase signaling, should be considered for these GISTs that have acquired a multitude of secondary mutations.

