MEETING HIGHLIGHT
Balancing efficacy and cardiovascular safety in chronic lymphocytic leukemia with acalabrutinib
Chronic lymphocytic leukemia (CLL) is a mature B-cell neoplasm characterized by an increased number of circulating clonal B-cells with typical morphology and immunophenotype.1 Accounting for 25% of all leukemias in adults, CLL is the most common leukemia in the Western world.1 To address the clinically heterogeneous and chronic nature of CLL, targeted therapies, such as Bruton tyrosine kinase (BTK) inhibitors, were developed to replace classic cytostatic agents and improve treatment outcomes.2 In the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Dr. John C. Byrd from The Ohio State University Comprehensive Cancer Center, United States and Dr. Jeff P. Sharman from The Willamette Valley Cancer Institute and The United States Oncology Network, shared the latest update on the efficacy and safety of a second generation BTK inhibitor, acalabrutinib, in patients with previously treated and untreated CLL.
Introduction of BTK inhibitors leads to a paradigm shift in treatment of CLL
Until recently, chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been the standard of care for young, fit CLL patients.2 However, chemoimmunotherapy is limited by the side effects including myelosuppression, infections and secondary malignancies.2,3 Moreover, the regimen has reduced activity in patients with genetic risk factors such as TP53 mutation, 17p deletion (del[17p]) and unmutated immunoglobulin heavy chain variable region (IGHV) status, prompting the need of a more targeted management approach for CLL.2
The introduction of BTK inhibitors, such as ibrutinib and acalabrutinib, began the era of CLL targeted therapies.2 Both Dr. Byrd and Dr. Sharman emphasized the importance of BTK as an essential protein for B-cell receptor (BCR) signaling and its critical role in CLL pathogenesis.4 By blocking the BCR signaling pathway, BTK inhibitors prevent the BCR signaling-dependent proliferation and survival of CLL cells.4 With an improved efficacy over the conventional chemoimmunotherapy in clinical trials, the latest National Comprehensive Cancer Network (NCCN) guideline now lists both acalabrutinib and ibrutinib as the “preferred regimen” (category 1) for patients with CLL without del(17p) or TP53 mutation regardless of age or comorbidities.5 For those less than 65-years-old without significant comorbidities, FCR is now considered an “other recommended regimen” (category 2A) instead of the “preferred regimen”.5 Under this guideline update, the CLL treatment paradigm has shifted away from conventional chemoimmunotherapy to targeted therapy.5,6
Acalabrutinib provided non-inferior survival benefit with lower cardiovascular toxicities comparing with ibrutinib in ELEVATE-RR
Although both acalabrutinib and ibrutinib are granted an equal level of recommendation in the NCCN guideline and share the same mechanism of action, they have different kinase selectivity profiles.5,7 Despite being the first irreversible BTK inhibitor available, ibrutinib also inhibits non-BTK, off-target kinases which may contribute to the adverse events (AEs) including rash, diarrhea, arthralgias or myalgias, atrial fibrillation (AF), and major hemorrhage that lead to treatment discontinuation.7-10 In contrast, acalabrutinib is a nextgeneration, irreversible BTK inhibitor with reduced off-target activity versus ibrutinib in vitro.4,7,11 Furthermore, Dr. Byrd commented that acalabrutinib’s higher selectivity for BTK can potentially result in a more favorable safety profile than ibrutinib without compromising efficacy in the treatment of CLL.4,7,11
The resulting clinical difference, particularly intolerability, was demonstrated in ELEVATE-RR, the first head-to-head trial comparing the safety and efficacy of acalabrutinib and ibrutinib in patients with previously treated CLL and del(17p) or del(11q).12 This phase 3, randomized, non-inferiority, open-label trial recruited 533 patients (median age of 66-years-old) and stratified them into subgroups by del(17p) status, Eastern Cooperative Oncology Group performance status (ECOG PS) and number of prior therapies.12 The patients were then randomized to receive oral acalabrutinib 100mg twice daily (n=268) or ibrutinib 420mg once daily (n=265) until progression or unacceptable toxicity.12
At a median follow-up of 40.9 months, acalabrutinib was non-inferior to ibrutinib with a median independent review committee (IRC)-assessed progression-free survival (PFS) of 38.4 months in both arms (HR=1.00; 95% CI: 0.79-1.27; Figure 1).12 Dr. Byrd also stated that the PFS was comparable between the two treatment arms across the prespecified subgroups.12 Median overall survival (OS) was not reached in either arm (HR=0.82; 95% CI: 0.59-1.15), with 63 (23.5%) deaths in the acalabrutinib arm and 73 (27.5%) deaths in the ibrutinib arm.12
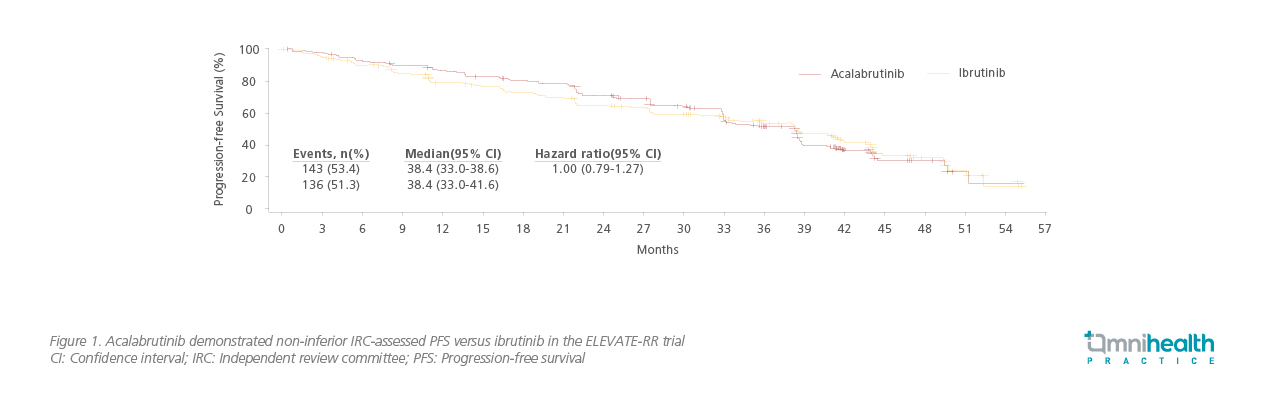
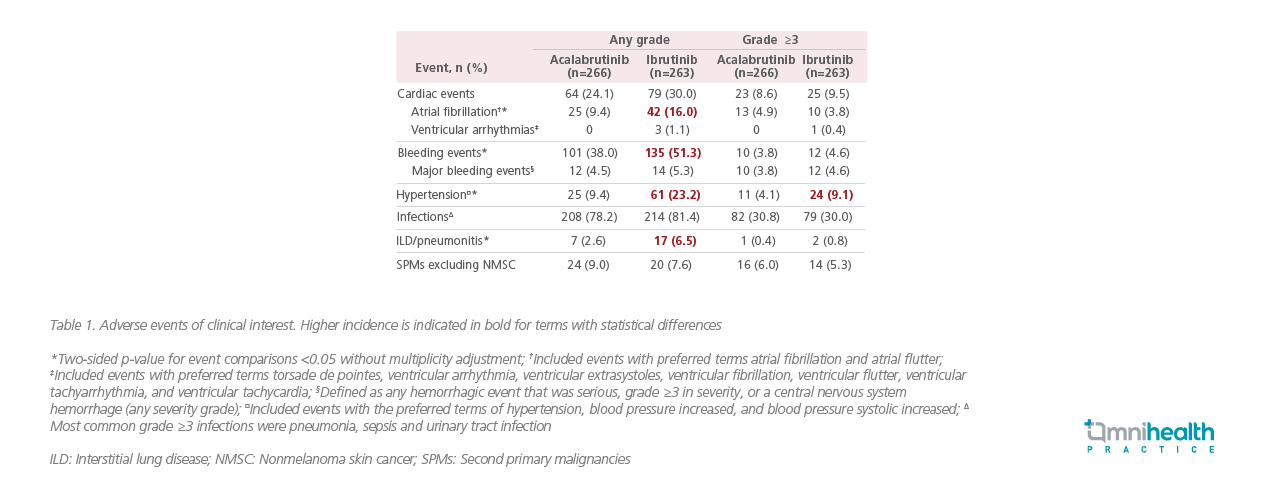
Dr. Byrd continued, “Non-inferiority studies typically demonstrated the benefit of acalabrutinib in other areas, lower frequencies of common AEs, grade ≥3 AEs, serious AEs, and treatment discontinuations due to AEs overall were observed with acalabrutinib in our study.” Notably, acalabrutinib was better tolerated than ibrutinib with statistically lower allgrade AF or flutter incidence (9.4% vs. 16.0%; p=0.02).12 Acalabrutinib also demonstrated superiority in the median onset time of AF or flutter (28.8 vs. 16.0 months) and AF- or flutter-related treatment discontinuation rate (0% vs. 16.7%).12 Among any-grade AEs of clinical interest, acalabrutinib was associated with a statistically lower (p<0.05) incidence of bleeding events, hypertension and interstitial lung disease (ILD) or pneumonitis when compared with ibrutinib, leading to treatment discontinuation in 14.7% and 21.3% of acalabrutinib-treated versus ibrutinib-treated patients, respectively (Table 1).12 With less common AEs observed, particularly the cardiovascular events, acalabrutinib is an equipotent and better tolerated BTK inhibitor as compared to ibrutinib for patients with previously treated CLL.12
When considering the selection between acalabrutinib and ibrutinib, based on ELEVATE-RR, Dr. Byrd stated that, “With similar efficacy and diminished toxicity, acalabrutinib would probably be the first choice [in previously treated CLL patients]. In patients who become intolerant to acalabrutinib, one might consider ibrutinib since the two agents have different chemical structures.”
In addition to previously treated CLL patients, acalabrutinib is also approved for treatment-naïve CLL patients based on the results of the ELEVATE-TN trial which compared acalabrutinib to the conventional chemoimmunotherapy.5,13 Previously, early results from this pivotal global, phase 3, multicenter, open-label study at a median follow-up of 28.3 months showed superior efficacy of acalabrutinib with or without obinutuzumab (A±O) than obinutuzumab plus chlorambucil (O+Clb) in patients with treatment-naïve CLL and a median age of 70-years-old (n=535).13
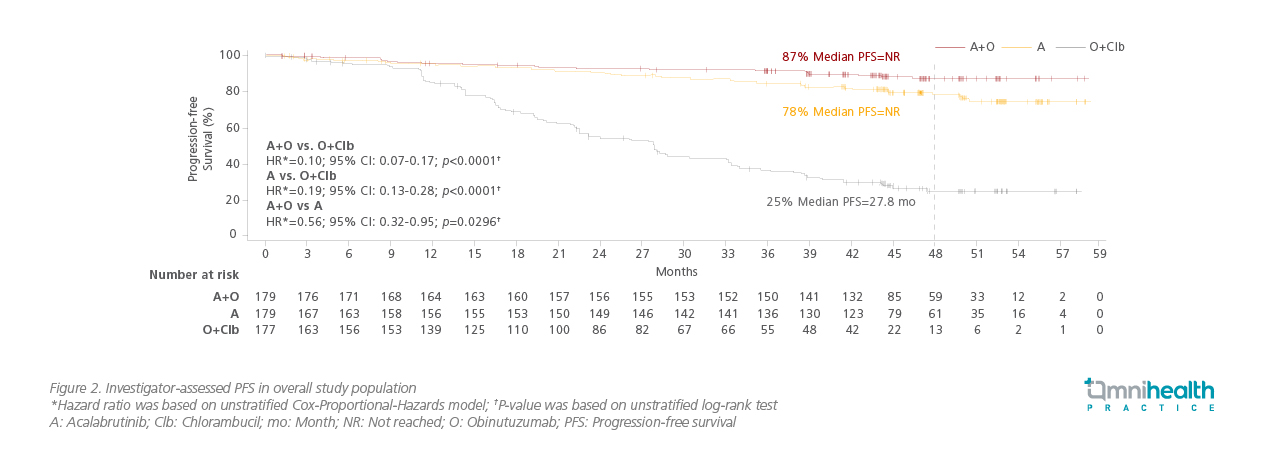
In the 4-year (median follow-up of 46.9 months) update presented in the 2021 ASCO Annual Meeting, over 69% of patients were still receiving A±O, and a total of 69 patients crossed over from O+Clb to acalabrutinib monotherapy.14 The median PFS remained significantly higher in the A±O arms versus O+Clb arm (not reached vs. 27.8 months; p<0.0001; Figure 2) and trended in favor of A+O over acalabrutinib monotherapy at 48 months (87% vs. 78%; HR=0.56; 95% CI: 0.32-0.95; p=0.0296).14 Acalabrutinib-containing treatments also prolonged PFS across high-risk genetic subgroups including patients with del(17p) and/or mutated TP53, noted Dr. Sharman.14 Significantly higher overall response rate (ORR) was observed with A±O (96.1% for A+O; 89.9% for acalabrutinib monotherapy) versus O+Clb (82.5%) as well (p<0.05).14
Most common AEs were generally unchanged from the interim report with no new toxicity observed.14 Despite longer treatment exposure, the incidences of cardiovascular events (AF and hypertension) and rates of treatment discontinuation were similarly low in both acalabrutinib containing (A±O) arms compared with previous results.14 Although neutropenia, fatigue and arthralgia occurred more frequently in the A+O arm versus the acalabrutinib monotherapy arm, both regimen were well-tolerated by treatment-naïve CLL patients.14 “Acalabrutinib with or without obinutuzumab demonstrated durable disease control, tolerability and flexibility to tailor treatment as a monotherapy or combination therapy in treatment-naïve CLL patients,” concluded Dr. Sharman.
Conclusion
As a second-generation BTK inhibitor, acalabrutinib provided non-inferior efficacy in PFS but lower AE incidence, particularly of the cardiovascular events, when compared with ibrutinib. As monotherapy or in combining with obinutuzumab, acalabrutinib demonstrated a durable PFS improvement over chemoimmunotherapy with maintained safety despite long-term treatment exposure. Given these promising results, acalabrutinib may serve as a potent and relatively tolerable option for patients with treatment-naïve and relapsed/refractory CLL.

