MEETING HIGHLIGHT
Local real-world study interim analysis demonstrates promising results: TTFields therapy on quality-of-life for newly diagnosed glioblastoma patients
With a rapidly deteriorating disease course, glioblastoma (GBM) is the most common and aggressive primary brain tumor with a typical 2-year and 5-year survival rate of 25% and 5-10%, respectively.1 Ever since the establishment of a standard-of-care treatment more than 15 years ago, comprising of surgical resection followed by adjuvant concurrent temozolomide chemo-radiotherapy (CCRT), little progress has been made.1 To address this unmet need, tumor-treating fields (TTFields) was introduced as a concurrent antimitotic treatment to CCRT and demonstrated a significant survival benefit among GBM patients worldwide as well as in Hong Kong.1 In the 27th Annual Scientific Meeting held by the Hong Kong Neurosurgical Society in December 2020, Dr. Peter Woo, Specialist in Neurosurgery, presented the interim analysis of a local study that demonstrated significant quality-of-life (QoL) and progression-free survival (PFS) benefits among newly-diagnosed GBM patients who received TTFields plus CCRT when compared to CCRT alone.
TTFields are addressing the unmet needs of GBM treatment
Despite the establishment of standard-of-care GBM treatment, the overall survival (OS) of patients in Hong Kong has not shown a significant improvement in the past 15 years with the 2-year survival rate remaining at 19%.1-3 To address this, TTFields was introduced as an additional antimitotic treatment utilizing non-invasive, low-intensity, intermediate-frequency alternative electric fields delivered by transducer arrays applied to the scalp.1 TTFields in addition to standard-of-care treatment significantly improved the 2-year (43% vs. 31%; p<0.001) and 5-year OS rate (13% vs. 5%; p=0.004) of GBM patients over CCRT alone.1 In the second-line treatment setting, Dr. Woo described a patient who achieved 9-months progression-free survival (PFS) with adjuvant TTFields, suggesting that TTFields beyond disease recurrence may also be beneficial. Moreover, TTFields did not demonstrate a significant negative influence on a patient’s health-related QoL.4 Given its efficacy and safety, a roundtable discussion at the 2015 American Society of Clinical Oncology (ASCO) meeting recognized TTFields plus temozolomide as the first major advance in the field of GBM therapy in a decade and should be considered for patients with newly diagnosed GBM.5 The latest National Comprehensive Cancer Network (NCCN) guidelines recommend TTFields as a first-line adjuvant treatment option with temozolomide CCRT for newly patients with newly diagnosed GBM with good functional performance scores regardless of age.6
Significant benefits of TTFields in the local real-world setting
To evaluate the efficacy and safety of TTFields plus CCRT among Hong Kong GBM patients, a local prospective study is currently being conducted to determine TTFields’s impact on QoL when compared to CCRT alone.3 Beginning from January 2019 to December 2023, up to 40 GBM patients from local neurosurgical and neuro-oncology centers are going to be recruited on a rolling basis.3 All patients are required to have a histological diagnosis of supratentorial GBM to be eligible for the study.3 Based on their baseline characteristics, patients will also be categorized into pre-specified subgroups according to their clinical, radiological or O6-methylguanine-DNA methyl-transferase promoter (pMGMT) methylation status.3
Prior to the study, Dr. Woo noted that patients need to complete CCRT followed by 3 months of TTFields, and then receive continuous TTFields treatment until significant adverse events occur or a change in treatment is requested.3 The primary endpoint is brain tumor-specific QoL at 6 months as graded by the European Organization for Research and Treatment of Cancer QoL questionnaire – brain tumor module (EORTC QLQ-C30/ BN20).3 Secondary endpoints are PFS, OS, caregiver strain index (CSI), treatment compliance, and treatment-related adverse effects.3
At the time of interim analysis, 14 patients completed at least 3 months of TTFields treatment and were compared against historical controls.3 The baseline patient characteristics, including a mean age of 56 years, a median Karnofsky Performance Status (KPS) score of 80, tumor pMGMT methylation status and extent of resection, were not statistically different from those in a historical control group.3
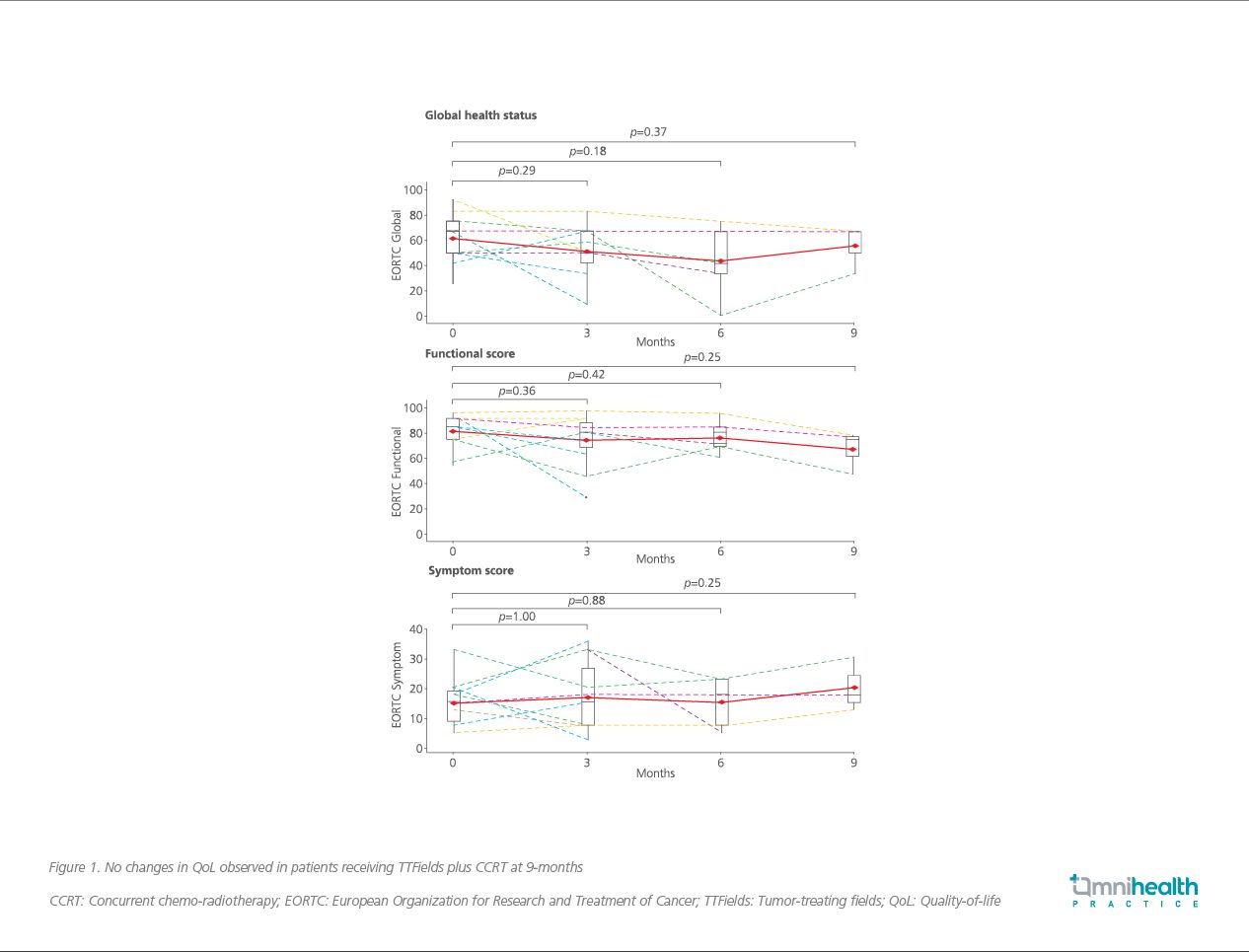
Compared to historical controls, TTFields plus CCRT showed comparable QoL across all EORTC domains at 6 months (51 vs. 48, 68 vs. 75 and 23 vs. 17, respectively; all p>0.05).3 In fact, no significant changes in QoL across the EORTC domains of global health status (p=0.37), functional score (p=0.25) and symptom score (p=0.25) were observed after 9 months of consecutive TTFields use (Figure 1).3 Notably, while the treatment period covered the summer months of Hong Kong which are known to be hot and humid, the QoL of these patients were preserved despite the need to constantly wear TTFields scalp arrays.3 “The QoL [of these patients] was remained essentially unchanged throughout the course of treatment,” commented Dr. Woo.
Whereas no statistical difference in PFS was observed across the entire study population at the interim analysis (13.3 months with TTFields plus CCRT vs. 6.7 months with CCRT alone; p=0.59), Dr. Woo remarked that TTFields plus CCRT showed significant PFS improvement among those with unmethylated pMGMT tumors when compared to CCRT alone (15.4 vs. 5.0 months; adjusted OR=0.47; 95% CI: 0.18-0.98; p=0.048).3 While OS data remained immature, 79% of patients receiving TTFields plus CCRT achieved 1-year survival and was comparable to the 73% observed in the multi-center, open-label, randomized clinical phase 3 EF-14 study.1,3 As such, Dr. Woo considered it to be reasonable to expect a significant survival benefit from TTFields when study patient recruitment is completed.
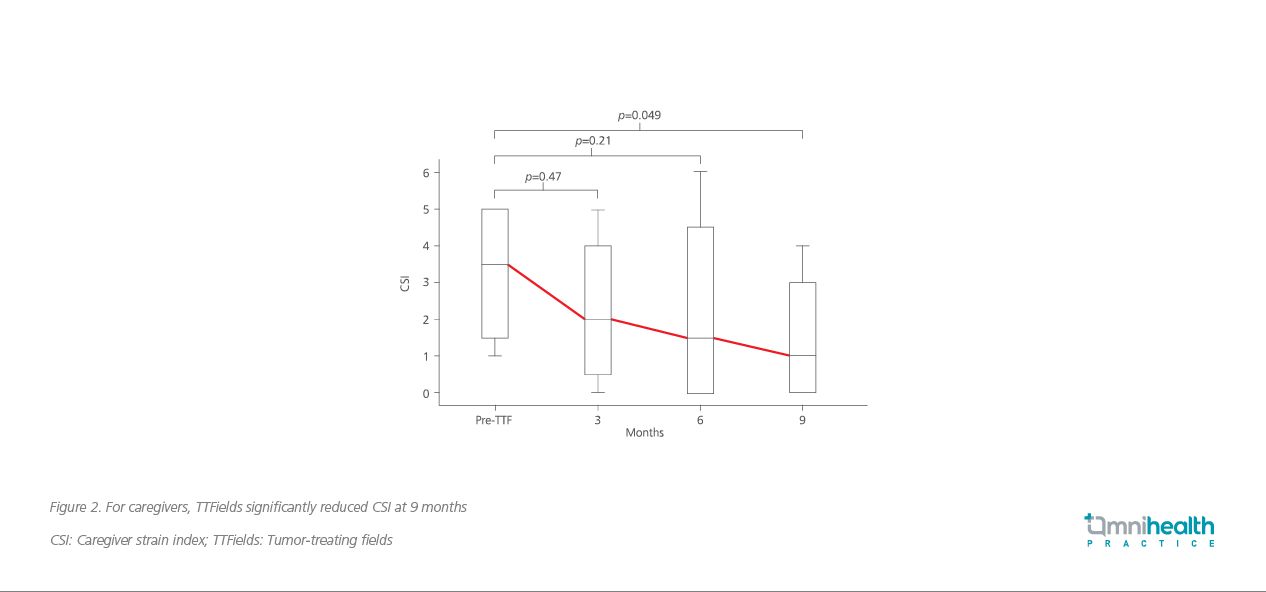
In addition, TTFields plus CCRT did not result in high caregiver burden, defined as a CSI of ≥7.3 The CSI, in fact, decreased from 3.5 to 1 by 9 months after TTFields initiation (p=0.049; Figure 2).3 A high compliance of 84% was also observed among patients, and the association between compliance and improved survival will be investigated when the study is completed.3
In terms of safety, 57% of patients experienced grade 1 scalp maculopapular contact dermatitis that was easily resolved with either array position adjustment or hydrocortisone/crotamiton topical creams.3 No seizures were observed with the use of TTFields.3
Positive forward expectation of TTFields based on the success of the global EF-14 study
While it is still too early to conclude a statistically significant survival benefit from the local study, Dr. Woo noted that TTFields plus CCRT already showed a significant survival benefit among GBM patients studied worldwide in the EF-14 study.1 In this phase 3 study, 695 patients at 83 sites across North America, Europe, the Republic of Korea and Israel were recruited.1 Patients were randomized after the end of CCRT in a 2:1 ratio to receive either maintenance temozolomide chemotherapy with or without the addition of TTFields.1
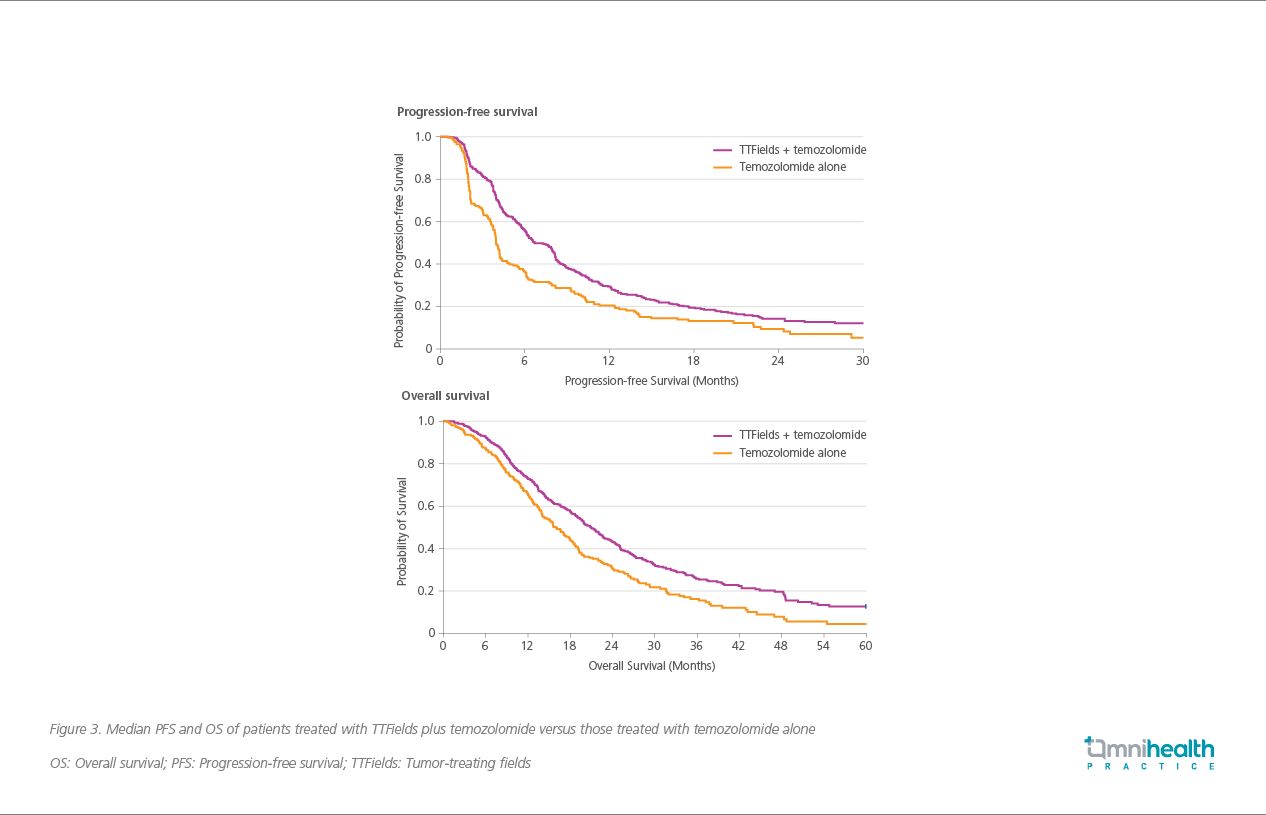
After a median follow-up of 40 months at a minimum of 24 months, patients treated with TTFields showed a significant improvement in median PFS (6.7 vs. 4.0 months; HR=0.63; 95% CI: 0.52-0.76; p<0.001) and median OS (20.9 vs. 16.0; HR=0.63; 95% CI: 0.53-0.76; p<0.001) when compared to temozolomide alone (Figure 3).1 Notably, a 1-year survival rate of 73% was observed among patients receiving TTFields plus temozolomide that is similar to the 79% observed in the local study population receiving TTFields plus CCRT.1,3 In addition, a subgroup analysis of the EF-14 study among the Asian subpopulation showed that TTFields significantly improved the 1-year survival rate to 96% versus 73% in those receiving temozolomide only (p=0.033).7 A post-hoc analysis further showed that the addition of TTFields can significantly improve OS when combined with second-line treatment after first disease recurrence versus chemotherapy alone (11.8 vs 9.2 months; HR=0.70; 95% CI: 0.48–1.00; p=0.049).8 a median follow-up of 40 months at a minimum of 24 months, patients treated with TTFields showed a significant improvement in median PFS (6.7 vs. 4.0 months; HR=0.63; 95% CI: 0.52-0.76; p<0.001) and median OS (20.9 vs. 16.0 months; HR=0.63; 95% CI: 0.53-0.76; p<0.001) when compared to temozolomide alone (Figure 3).1 Notably, a 1-year survival rate of 73% was observed among patients receiving TTFields plus temozolomide that is similar to the 79% observed in the local study population receiving TTFields plus CCRT.1,3 In addition, a subgroup analysis of the EF-14 study among the Asian subpopulation showed that TTFields significantly improved the 1-year survival rate to 96% versus 73% in those receiving temozolomide only (p=0.033).7 A post-hoc analysis further showed that the addition of TTFields can significantly improve OS when combined with second-line treatment after first disease recurrence versus chemotherapy alone (11.8 vs. 9.2 months; HR=0.70; 95% CI: 0.48-1.00; p=0.049).8
Compared to the local study, TTFields plus temozolomide in the EF-14 study showed a statistically significant survival benefit regardless of pMGMT methylation status, extent of resection, patient race, age, gender and functional performance status.1,3 Particularly, patients with methylated pMGMT demonstrated a greater OS benefit when compared to those with unmethylated pMGMT (HR=0.50; 95% CI: 0.41-0.62; p<0.001), supporting pMGMT methylation status as a strong prognostic biomarker for GBM patients treated with temozolomide.9 Interestingly, a PFS improvement was only observed among those with unmethylated pMGMT in the local study, and Dr. Woo attributed this difference to the small patient group size and early survival data which can be clarified when data accrual is complete.3
In terms of safety, the addition of TTFields was not associated with any significant increased risk of systemic adverse events when compared to temozolomide alone (48% vs. 44%; p=0.58).1 While more than half of the patients in the TTFields group (52%) experienced mild to moderate skin irritation, only 2% experienced severe (grade 3) skin involvement.1 Dr. Woo compared this safety result to the local study where 57% of patients experienced grade 1 skin irritation that was easily resolved with no severe manifestations observed.3 Despite a high prevalence of skin irritation, 75% of patients in the EF-14 study achieved ≥75% treatment adherence and was comparable to the 84% compliance rate observed in the local study.1,3
Although the pivotal EF-14 study demonstrated significant survival benefit with TTFields, controversies exist regarding how the majority of trial subjects were recruited from tertiary care centers.1 As more frequent care and support from healthcare professionals are available at these tertiary care centers, patients in the EF-14 study may have improved survival outcome and may not truly reflect real-world experience. On the other hand, the local study included patients who were utilizing TTFields in a public healthcare system.3 Under this real-world use of TTFields, similar 1-year survival rates and PFS among GBM patients with unmethylated pMGMT were also observed even when only 6 months of interim data was accounted for.3 With the interim local study results showing great promise under a real-world setting, Dr. Woo concluded that “Indeed, in the local study, while it is still too early to determine the survival benefit [of TTFields], a similar trend in OS and PFS to EF-14 was observed with QoL comparable to patients receiving CCRT alone.”
Conclusion
TTFields therapy is a locally novel adjuvant treatment modality for GBM patients and has so far demonstrated no significant worsening of QoL with a similar 1-year survival rate and PFS among GBM patients with unmethylated pMGMT when compared to CCRT alone. While it is still too early to determine the OS benefit, the local study data appears promising, delivering a benefit similar to that observed in the phase 3 EF-14 study. Caregiver stress was significantly reduced by 9 months and high treatment compliance was observed. TTFields may become a standard-of-care for treating local GBM patients in the near future.

