CONFERENCE UPDATE: ENDO 201
Drugs in the pipelines for managing childhood and adolescent severe obesity
The increasing prevalence of overweight and obesity in children and youths leads to the concern of developing metabolic morbidities throughout adulthood and increasing healthcare burden.1 While pharmacotherapy is an effective option to control weight gain, current clinical practice guideline recommends the initiation of pharmacotherapy among children or adolescents only after vigorous behavioral changes with ongoing lifestyle modification throughout the course of treatment.2,3
Currently, the United States Food and Drug Administration (FDA) has approved various anti-obesity drugs for pediatric patients including pancreatic lipase inhibitor orlistat, glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide and norepinephrine reupdate inhibitor phentermine. Where the efficacies of orlistat and liraglutide were validated in randomized clinical trials, phentermine was evaluated in a retrospective observational study (Table 1).4-7
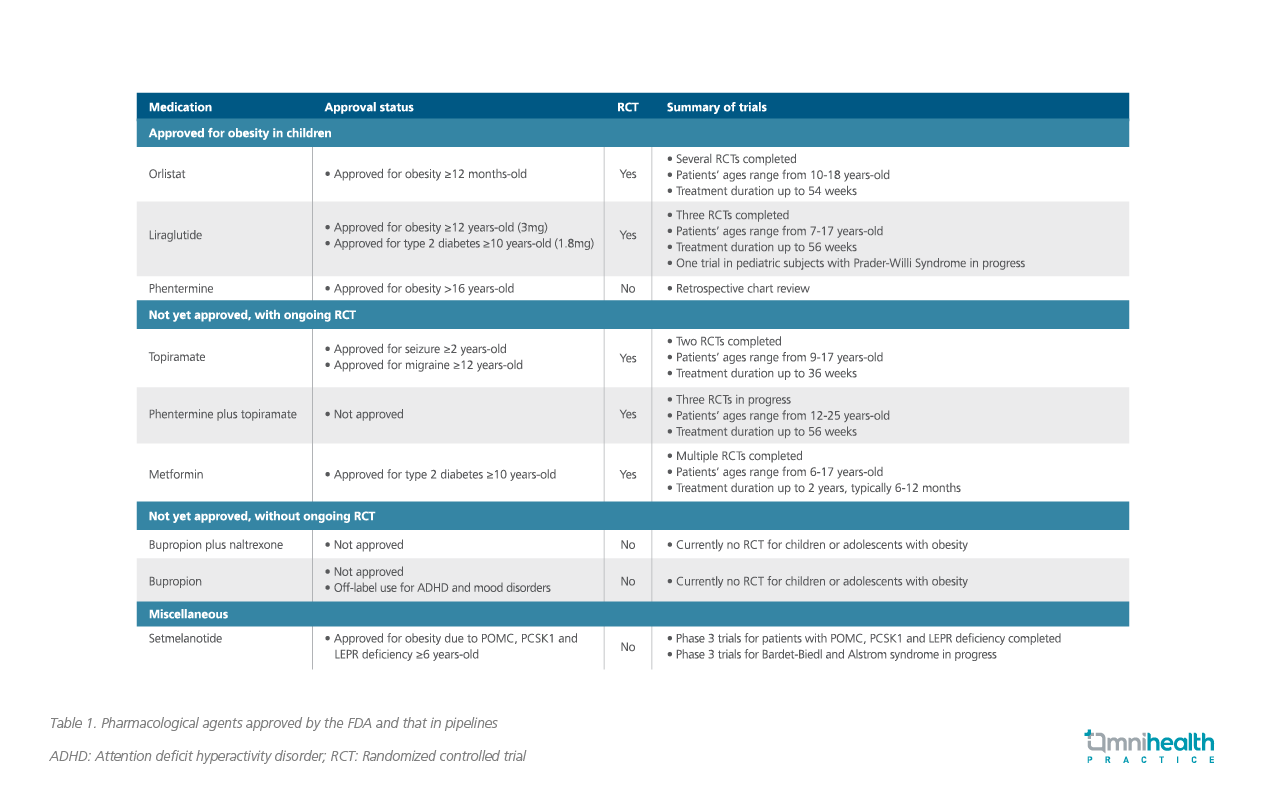
In addition to the approved medications, Dr. Ania Jastreboff, Associate Professor of Internal Medicine, Endocrinology and Metabolism from the Yale University School of Medicine, United States, commented that topiramate monotherapy or in combination with phentermine as well as metformin are associated with weight loss and have been used off-label to treat obesity-related morbidities among pediatric patients. Several ongoing randomized clinical trials (RCTs) are now under study to evaluate the efficacy of topiramate and metformin on weight control in patients between 12 and 24-years-old with results expected to be available by July 2021 and August 2025, respectively.
When selecting patients for optimal treatment, Dr. Jastreboff concluded that “Pharmacotherapy is indicated in all children with type 2 diabetes and in selected adolescents with obesity.” While more pharmacological agents being available soon, Dr. Jastreboff emphasized the need of further mechanistic trials to elucidate the pathophysiology and mechanisms of these drugs. “Long-term clinical trials should be conducted to inform the long-term safety and effectiveness of various pharmacotherapies,” noted Dr. Jastreboff.

