CONFERENCE UPDATE: NCCN 2021
More radiotracers approved for PSMA PET/CT scan in the metastatic castration-resistant prostate cancer diagnosis
Radiologic imaging has been a cornerstone in guiding treatment approaches for metastatic castration-resistant prostate cancer (mCRPC). However, conventional bone scintigraphy using 99Tc-MDP is limited by its low sensitivity towards prostate cancer cells and many mCRPC cases may remain undetected by this conventional approach.1 In a clinical case example shared by Professor Sandy Srinivas, Stanford University School of Medicine, United States, conventional 99Tc -MDP bone scan could only detect oligometastases in a 74-year-old man with biochemical recurrence of prostate cancer, while the more recent 18F positron emission tomography (PET)/computed tomography (CT) revealed extensive polymetastases in this patient. In this connection, Prof. Srinivas urged that bone scans may not be ideal in evaluating mCRPC patients and the more specific prostate-specific membrane antigen (PSMA) PET/CT should be adopted.
In the recent years, several radiotracers for PET/CT that specifically bind to prostate-specific antigens (PSA) were approved by the Food and Drug Administration (FDA) (Table 1).2 These radiologically labeled small molecules have short half-lives, ranging from 20 minutes for 11C to 68 minutes for 68G and 110 minutes for 18F, and can be excreted through urine (18F and 68G) or the hepatobiliary route (11C).2
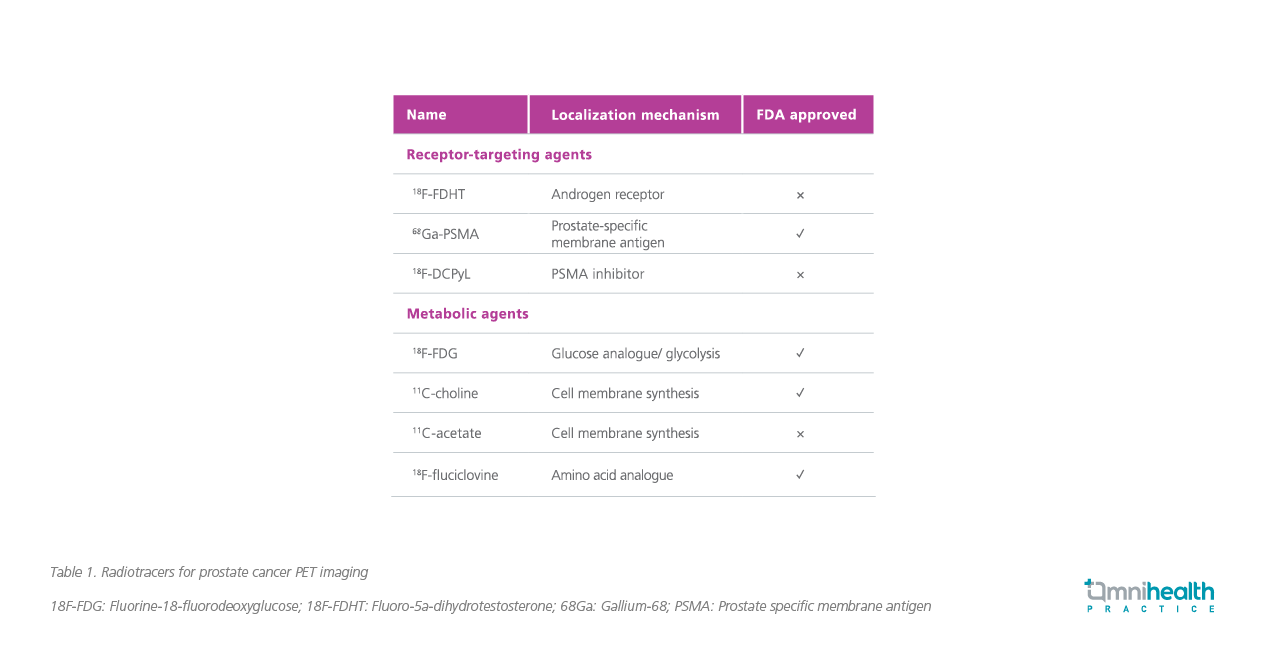
Fluciclovine is one such radiolabel that is commonly ligated to fluoro- 5a-dihydrotestosterone (18F-FDHT) or fluorodeoxyglucose (18F-FDG) and can be actively taken up by prostate cancer cells via upregulated amino acid transporters such as ASCT2 and LAT1.3 Notably, fluciclovine is well-tolerated and is able to detect local and distant prostate cancer recurrence even among patients with lowest quartile for baseline PSA level of ≤0.79ng/ml with a detection rate of 41.4%.3
In the phase 2/3 prospective multicenter OSPREY study, the fluciclovine-ligated radiolabel 18F-DCFPyL PET/CT demonstrated a positive predictive value (PPV) of 86.7% and a negative predictive value (NPV) of 83.2% in high-risk prostate cancer patients who underwent radical prostatectomy with pelvic lymphadenectomy.4 Similarly, 18F-DCFPyL PET/CT achieved a median sensitivity and PPV for extra-prostatic lesions of 95.8% and 81.9%, respectively, for patients with suspected recurrent/metastatic prostate cancer.4 Given its reliability in detecting sites of disease as well as staging patients for nodal or distant metastases, PSMA PET/CT, such as 18F-DCFPyL PET/CT, should be used to support decision making when managing prostate cancer patients, noted Prof. Srinivas.4
In practice, these diagnostic PSMA PET/CTs can be combined with available therapeutics options to establish a “theranostics” approach. Notably, many prostate cancer patients harbor BRCA mutations and are suspectable to the synthetic lethal targeting of the single-strand DNA break repair pathway by poly (ADP-ribose) polymerase (PARP) inhibitors.5 Whereas conventional biopsy at the bone lesion sites is difficult to perform and complicates the confirmation of genetic aberrations, Prof. Srinivas noted that recent advances in liquid biopsy as well as the approval of multiple radiotracers have enabled a more accurate detection, diagnosis and treatment of patients with prostate cancer. By combining improved imaging modalities with effective treatment options, the theranostic approach can improve the survival of mCRPC patients while maintaining their quality of life. “The goal of the theranostic approach is to deliver effective, life-prolonging therapy to patients with mCRPC while balancing the longevity with good quality of life,” concluded Prof. Srinivas.

