CONFERENCE UPDATE: EHA 2025
Talquetamab + teclistamab yields deep and durable responses in EMD-positive RRMM: Results from the phase 2 RedirecTT-1 trial
STUDY DESIGN
Extramedullary disease (EMD) in multiple myeloma (MM) is associated with a poor prognosis, with patients being approximately 87% less likely to respond to standard treatments compared to those without EMD.1 Previous pivotal studies have shown modest efficacy of monotherapy bispecific antibody (BsAb) in this setting, where patients with EMD treated with talquetamab (antiGPRC5D) and teclistamab (antiBCMA) monotherapies have both exhibited overall response rates (ORR) of approximately 43%.1
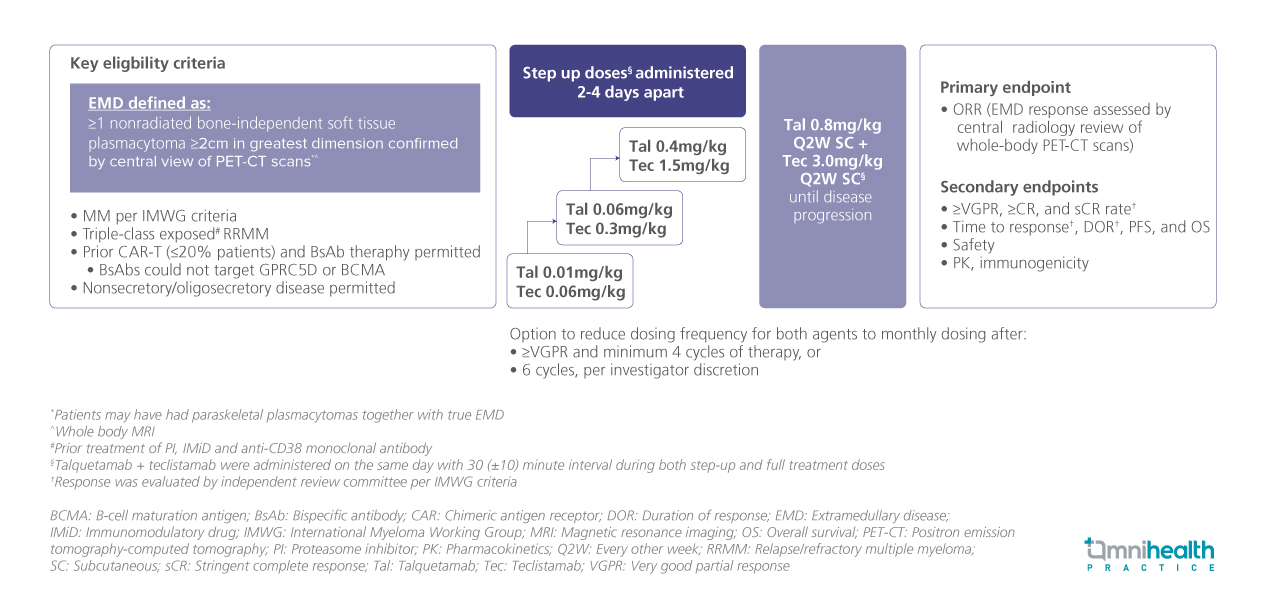
Building on preliminary data from the RedirecTT-1 phase 1 trial, the phase 2 RedirecTT-1 trial enrolled patients aged ≥18 years with measurable MM with true EMD, defined as ≥1 non-radiated bone-independent soft tissue plasmacytoma ≥2cm in greatest dimension as confirmed by central review of positron emission tomography–computed tomography (PET-CT) scans.1 Patients with triple-class-exposed relapsed/refractory MM (RRMM) were included.1 Prior CAR-T or BsAb therapy (not targeted at GPRC5D or BCMA) and those with non-secretory or oligosecretory disease were permitted.1 Participants received maintenance therapy with talquetamab (0.8mg/kg) + teclistamab (3.0mg/kg), administered subcutaneously every two weeks, following a step-up dosing regimen, and continued until disease progression.1
A total of 90 patients were enrolled.1 Of these, 39% had non-secretory/oligosecretory disease, 20% had received prior anti-BCMA CAR-T therapy, and 9% had prior BsAb exposure.1 Notably, 84% were triple-class refractory, and 36% were penta-drug refractory, underscoring a cohort with difficult-to-treat disease.1 The median follow-up duration was 12.6 months.1
The primary endpoint was the overall response rate (ORR) determined based on EMD response assessed by central radiology review of whole-body PET-CT scans.1 The secondary endpoints presented included time to response, duration of response (DOR), median progression-free survival (mPFS), and overall survival (OS).1
FINDINGS
|
|
|
|
|
|
|
|
|
|
|
|
|

