FEATURES
Sequencing retreatment with anti-CD38 mAb in heavily pretreated RRMM patients
Patients with multiple myeloma (MM) are highly susceptible to developing relapsed and refractory (RR) disease towards lenalidomide and proteasome inhibitors (PIs) and require new active treatments at relapse.1 As such, novel treatment options like isatuximab, a monoclonal antibody (mAb) that targets CD38, have been developed.1,2 At the 2023 Annual Scientific Meeting of the Hong Kong Society of Myeloma, Professor Yong, Kwee from University College London shared the latest data on the encouraging response of isatuximab-based regimens among patients with lenalidomide-refractory disease and high-risk cytogenetics. She also talked about the sequencing of anti-CD38 mAb use based on a real-world study that investigated the efficacy of isatuximab after daratumumab treatment, highlighting the importance of a treatment-free interval between the two regimens.
Isatuximab as a potential candidate in RRMM treatment
MM can be a chronic threat to patients’ health due to relapse being an inevitable feature of the disease.1 In the management of MM, lenalidomide forms the backbone of many current front-line regimens regardless of transplant eligibility.3,4 However, almost all MM patients eventually become refractory to lenalidomide.1 As such, novel treatment options for MM with alternative mechanisms of action have emerged. Prof. Yong noted that there are many second-line options for lenalidomide-refractory patients, including proteasome inhibitor (PI)-based, pomalidomide-based, and anti-CD38 mAb-based treatments, and that regimens not used in earlier lines may be rechallenged.
Isatuximab is an anti-CD38 monoclonal antibody (mAb) that binds to a specific epitope of CD38 and exhibits anti-tumor properties through a number of unique biological mechanisms, including antibody-dependent cellular-mediated cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, and direct induction of apoptosis without cross-linking.1,2 Furthermore, isatuximab possesses a dose-dependent inhibition property against CD38 ectoenzyme activity, which has key roles in cellular functions and contributes to immune system evasion of tumor cells.1 Preclinical studies have demonstrated a favorable efficacy in tumor regression when isatuximab was combined with immunomodulatory agents or PIs.2
According to both the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines on MM, isatuximab-based regimens are one of the combination therapies indicated for lenalidomide-refractory, or lenalidomide-refractory and bortezomib-refractory patients.3,4 Isatuximab plus carfilzomib/dexamethasone (Isa-Kd) is recommended at first relapse while isatuximab plus pomalidomide/dexamethasone (Isa-Pd) is recommended in a similar group of patients at the second or subsequent relapses.3,4
Clinically meaningful and sustained response of Isa-Pd in lenalidomide-refractory MM patients reported in the ICARIA-MM study
Prof. Yong presented findings of the ICARIA-MM study, which was the first phase 3 study that investigated the clinical efficacy of Isa-Pd among patients with RRMM.1 In this study, 307 patients with RRMM who received ≥2 lines of treatment and exhibited refractory towards lenalidomide and PIs were randomized 1:1 to receive Isa-Pd (n=154) or Pd only (n=153) until disease progression, unacceptable toxicities, or patient withdrawal.1
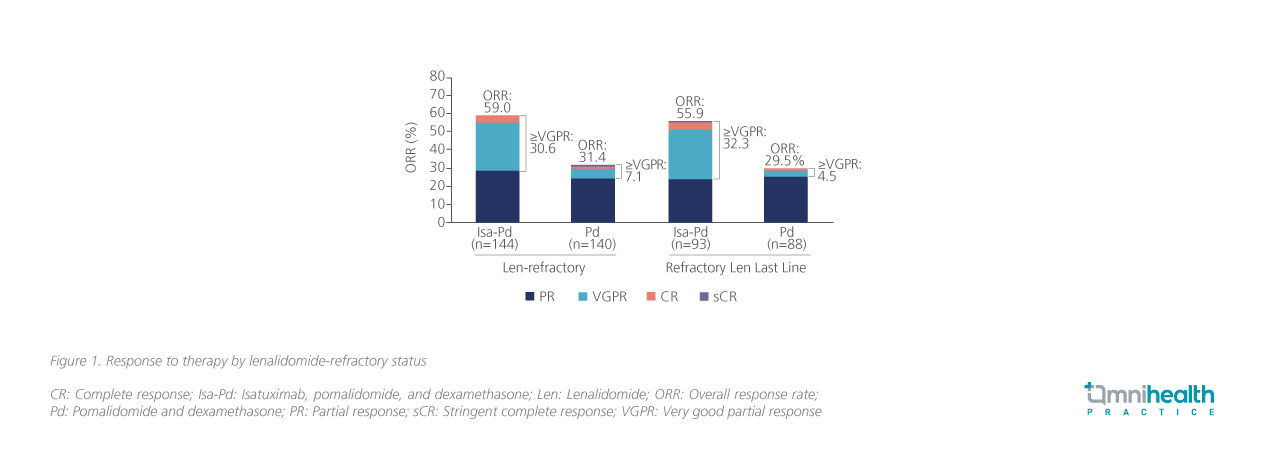
At a median follow-up of 11.6 months, the median progression-free survival (PFS) of the Isa-Pd arm was significantly longer than that of the Pd-only arm, indicating an approximately 40% reduced risk of progression and death (11.5 vs. 6.5 months; HR=0.596; 95% CI: 0.44-0.81; p=0.001).1 In addition, the overall response rate (ORR) of the Isa-Pd arm was also significantly higher than that of the Pd-only arm (60% vs. 35%; OR=2.795; 95% CI: 1.75-4.56; p<0.0001).1 A subsequent subgroup analysis revealed that compared to the Pd-only arm, a higher ORR was observed in Isa-Pd arm among patients who were lenalidomide-refractory (59.0% vs. 31.4%) and those who were lenalidomide-refractory at last line (55.9% vs. 29.5%), with the proportion of patients who exhibited very good partial response (VGPR) maintained at 30.6% and 32.3% respectively (figure 1).5
The desirable clinical responses of Isa-Pd were retained even 3 years after treatment.6 At a median follow-up of 35.3 months, the ORR was reported at 63.0% vs. 33.3% with Isa-Pd and Pd-only respectively, with the proportion of patients in the Isa-Pd arm who exhibited VGPR being tripled that of the Pd-only arm (38.3% vs. 10.5%).6
Consistent clinical benefits of Isa-Pd in MM patients with high-risk cytogenetics
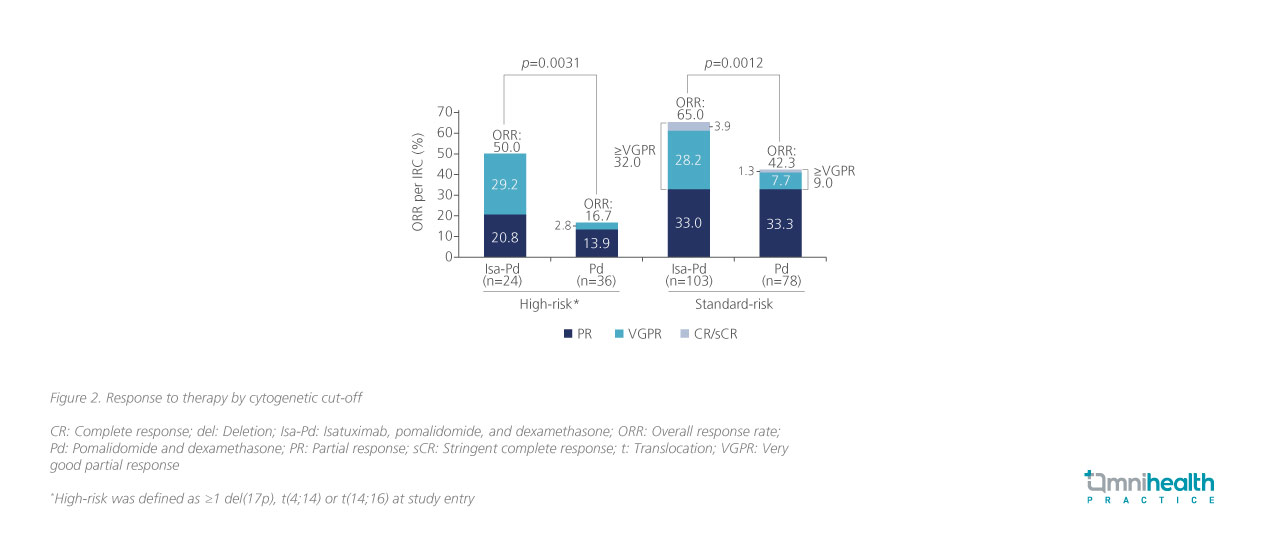
In another subgroup analysis of ICARIA-MM, which explored the clinical efficacy of Isa-Pd among MM patients with high-risk cytogenetics, the Isa-Pd regimen demonstrated comparable clinical benefits between the standard-risk and high-risk patient subgroups.7 Amongst the MM patients with high-risk chromosomal abnormalities including del(17p), t(4;14), or t(14;16) mutations (n=60), patients who received Isa-Pd treatment (n=24) exhibited a significantly higher ORR compared to those who received Pd only (n=36) (50.0% vs. 16.7%; p=0.0031) (figure 2).7 The survival outcomes associated with Isa-Pd were also consistent regardless of chromosomal abnormalities.7 When stratified by cytogenetic risk, the PFS benefit induced by Isa-Pd among high-risk patients (HR=0.66; 95% CI: 0.33-1.28) was comparable with standard-risk patients (HR=0.62; 95% CI: 0.42-0.93).7
Robust data for alternative isatuximab-based triplet regimen in the IKEMA trial
Aside from Isa-Pd, Isa-Kd, the sister regimen of Isa-Pd has also shown remarkable clinical benefits among high-risk patients with RRMM.2 To demonstrate its efficacy, Prof. Yong presented the recent findings of the IKEMA trial, a phase 3 open-label study that evaluated the clinical efficacy of Isa-Kd among early-relapse MM patients.2 In this study, 302 patients with relapsed or refractory MM with 1 to 3 prior lines of therapy were randomized 3:2 to receive Isa-Kd (n=179) and Kd (n=123) until disease progression, unacceptable toxicities, or patient withdrawal.2
Significant improvements in PFS were reported in the Isa-Kd arm at a median follow-up of 20.7 months, resulting in a 47% risk reduction in progression or death (HR=0.53; 99% CI: 0.32-0.89; p=0.0007).2 This survival benefit was maintained at a follow-up of 44 months, where there was a 42% reduced risk of progression or death (HR=0.58; 95% CI: 0.42-0.79).9 Despite the similar ORR between the Isa-Kd and Kd-only arms (86.6% vs. 83.7%), the benefits of Isa-Kd over Kd monotherapy were more apparent as patients who received Isa-Kd had a higher achievement rate of VGPR (72.6% vs. 56.1%) and stringent complete or complete responses (sCR/CR) (44.1% vs. 28.5%).2,9
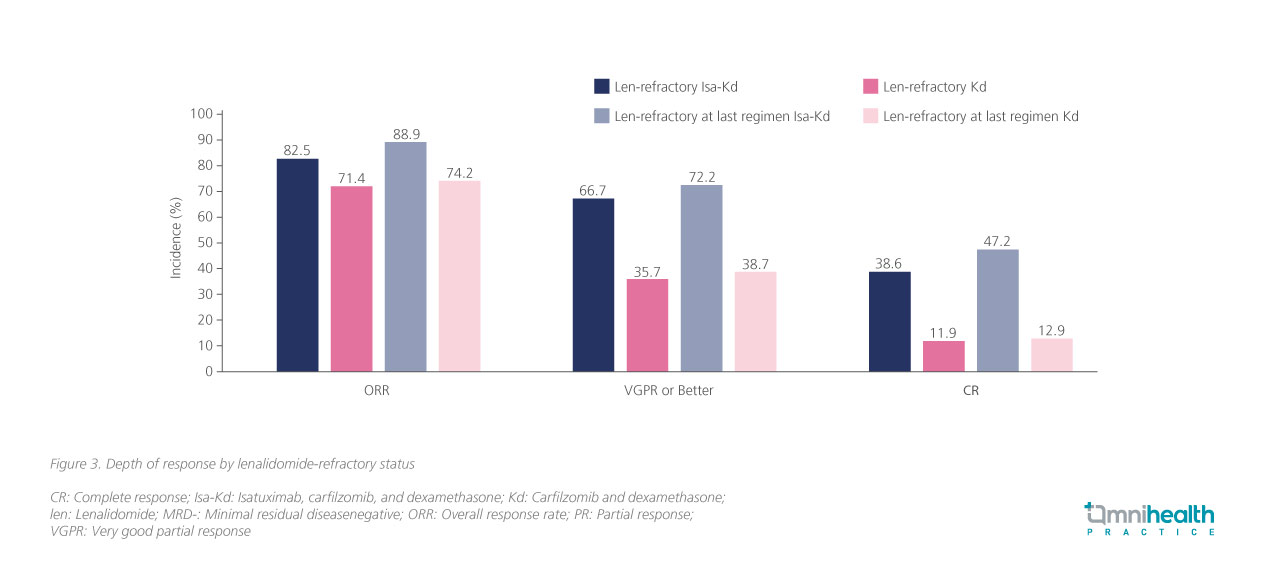
Echoing the exploratory data of the ICARIA-MM study, the ORR data among patients in IKEMA also indicated consistent clinical efficacy of Isa-Kd regardless of patients’ lenalidomide-refractory status (figure 3).8
Additionally, at the median follow-up of 44 months, the safety profile was similar to previous analyses where the treatment discontinuation rate of Isa-Kd was even lower than that of Kd alone (12.4% vs. 18.0%). Prof. Yong also highlighted the similar incidence of cardiac failure (8.5% vs. 7.4%) and grade ≥3 cardiac failure (4.5% vs. 4.1%) between the Isa-Kd arm and Kd-only arm, suggesting that the addition of isatuximab did not incur any additional cardiotoxicity, and that a triplet therapy enables more opportunities for dose adjustment if so required.9
Isatuximab in real-life post-daratumumab populations
Prof. Yong reflected that while isatuximab-based regimens have been proven to be an efficacious second-line option for treating RRMM, the management of patients in real life may be more complex as they are often heavily pretreated prior to the use of isatuximab-based regimens, with other anti-CD38 mAbs such as daratumumab. She also raised the question of how prior daratumumab treatment may affect patients’ response to isatuximab-based regimens and noted the need for a washout period between the 2 treatments.
In a retrospective study, patients with RRMM from 4 US academic institutions (Medical College of Wisconsin, University of Arkansas for Medical Sciences, Memorial Sloan Kettering Cancer Center, and MD Anderson Cancer Center) who had ≥1 cycle of daratumumab therapy and subsequently had isatuximab-based regimen were analyzed.10 The study reported that these patients were heavily pretreated with a median of 6 prior lines of therapy (range: 1-18) and that 64.4% of patients had an interval of >6 months between daratumumab and isatuximab therapy. A clinical benefit rate (CBR) (defined as the percentage of patients who achieved a sCR, CR, VGPR, PR, or MR) of 44% was attained among the study population, with a significantly higher CBR being observed in patients with a >6 months washout period (n=38) compared to those who received isatuximabbased regimens ≤6 months (n=21) from the last daratumumab dose (58% vs.19%; p=0.004).10 Results from the multivariate analysis also showed that a washout period of >6 months is independently associated with better CBR response (OR=0.23; 95% CI: 0.06-0.88; p=0.032).10
Based on the findings of this study, it was speculated that the benefit of the washout period stemmed from possible antigen downregulation or impaired effector cell function from prior anti-CD38 mAb therapy, but Prof. Yong asserted that the precise mechanisms behind these interactions warrant further investigation.
Conclusion
With the increasing prevalence of lenalidomide-refractory patients, the role of anti-CD38 mAbs has become increasingly important.1 High rates of ORR sustained in lenalidomide-refractory patients of the Isa-Pd and Isa-Kd regimens from the ICARIA-MM and IKEMA trials respectively have demonstrated its value in later-line treatment in RRMM patients as well as patients with high-risk cytogenetics.1,2,6,9 When used in real-life practice, where patients may be heavily pre-treated with other anti-CD38 mAb regimens, Prof. Yong reminded us that a washout period of >6 months between daratumumab-based and isatuximab-based regimens may improve the response to isatuximab-based regimens.

