Preserving bone integrity with the appropriate choice of hypoglycemic agents
Fragility fractures are increasingly recognized as a relevant complication of both type 1 and 2 diabetes mellitus (T1DM/T2DM).1 Compared to the general population, T1DM and T2DM patients have a significantly higher fracture risk in both males and females at all ages with the difference increasing further with age.1-3 However, while evidence from both preclinical studies and human clinical trials showed a strong correlation between glucose levels and bone metabolism, hypoglycemic agents could further disturb bone architecture and exaggerate fracture risk.1-7
Professor Paolo Pozzilli, Professor of Endocrinology and Diabetes, Campus Bio-Medico University of Rome, Italy, explained that chronic hyperglycemia can cause oxidative stress, inflammation, production of reactive oxygen species and advanced glycation end products (AGEs) that cause organ damage and reduce bone strength. In particular, the accumulation of AGEs in diabetic bone collagen can reduce bone material properties and increase the susceptibility to fracture.1 Also, both AGEs and hyperglycemia can directly inhibit bone formation via the suppression of osteoblast function.1
In practice, Prof. Pozzilli highlighted that “We have a number of [antidiabetic] drugs that are used which can indeed affect either bone formation or bone resorption.” In particular, the pathogenesis of diabetes can disrupt bone architecture and the choice of hypoglycemic agents is clinically important to avoid further disturbing bone homeostasis (Table 1). Among the many available options, metformin is the most common antidiabetic drug worldwide. Previously, cell culture of the murine pre-osteoblast cell line MC3T3E1 suggested that metformin could enhance osteoblast proliferation through the upregulation of adenosine 5-monophosphate-activated protein kinase (AMPK) and Runt-related transcription factor 2, a regulator of osteoblastic bone formation. At the same time, metformin also reduced bone resorption by modulating the receptor activator of nuclear factor kappa B (RANK)/receptor activator of nuclear factor kappa B ligand (RANKL)/osteoprotegerin pathway in MC3T3E1 cells. Despite the wide adoption of metformin, Prof. Pozzilli commented that further clinical studies would be needed to determine the efficacy of metformin in preventing bone fractures among diabetic patients.
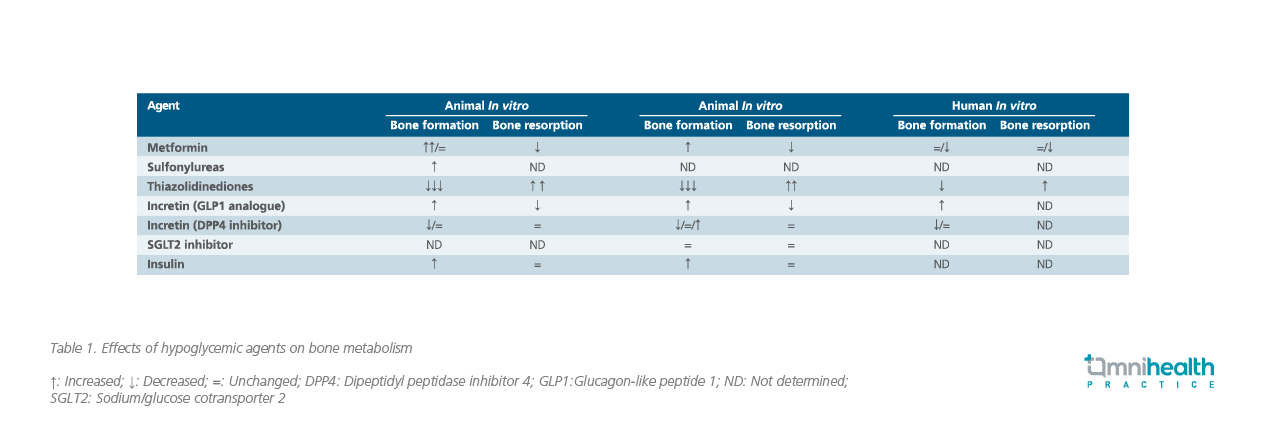
Other than metformin, antidiabetic agents include sulfonylureas, incretin agents and insulin are also available for treating diabetes. In an earlier study, glimepiride, a third-generation sulfonylurea, was shown to upregulate the phosphoinositide-3-kinase (PI3K)/Atk pathway in rat osteoblasts which increased the proliferation and differentiation of blast cells. However, further data to support the effect of sulfonylureas on bone metabolism is limited and more studies would be required to confirm the underlying mechanism. On the other hand, incretin agents such as glucagon-like peptide 1 (GLP-1) analogue could increase bone formation and reduce bone resorption while dipeptidyl peptidase-4 (DPP4) inhibitors may affect the bone metabolism and impair the bone quality (i.e., trabecular architecture) as shown in DPP4-knockdown diabetic mice.8
While insulin has an anabolic effect on bone and may improve bone formation, both preclinical and clinical evidence suggested that the insulin sensitizer thiazolidinedione is associated with an increased risk of fracture. In fact, treatment of Wistar rats with the thiazolidinedione rosiglitazone revealed significant increase of fat marrow volume and extended bone erosion by week 12, attributed to the increased proliferation of osteoclasts leading to the active breakdown of osteocytes.8
Sodium glucose co-transporters 2 inhibitor (SGLT2i) is a new class of hypoglycemic drug with limited studies suggesting neutral or beneficial effect on bone architecture. In murine models, exposure to the SGLT2i dapagliflozin resulted in increased tissue mineralization and trabecular bone accretion without a reduction in bone mineral density.8 Notably, Prof. Pozzolli highlighted that the long-term use of dapagliflozin for two years in T2DM patients did not result in any significant change in bone turnover biomarkers.8 Similarly, bone resorption markers including parathyroid hormones remained unchanged following the treatment of animals using another SGLT2i canagliflozin.8 When considering antidiabetic treatment, Prof. Pozzilli concluded that hypoglycemic agent SGLT2i does not appear to affect the bone resorption or formation, supporting its benefit on bone architecture.

