FEATURES
Raising the bar in AD care: Delivering deep and durable responses with lebrikizumab
|
Highlights
|
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease that significantly affects patients with persistent itching, skin lesions, pain, and reduced quality of life.1,2 Advancements in our understanding of AD pathophysiology have paved the way for targeted biologic therapies, transforming treatment strategies.3 At the Global Chinese Dermatology Summit 2025, Professor Leon Kircik, Clinical Professor of Dermatology at Icahn School of Medicine, shared his expertise across two sessions. In the symposium “The Impact of Advanced Therapy on Atopic Dermatitis: Enhancing Patient Experience in Treatment Strategies”, he examined the evolving treatment paradigm in AD and the role of interleukin (IL)-13 inhibition. In a separate oral poster presentation, “Raising the Bar of Efficacy in Atopic Dermatitis: Lebrikizumab Maintains Depth of Response Over 3 Years in Week 16 Responders”, Prof. Kircik highlighted the long-term clinical evidence for lebrikizumab, underscoring its potential to redefine durable disease control in AD.
From partial relief to precision targeting: IL-13 at the core of AD
Poorly controlled AD carries consequences that extend well beyond the skin. Persistent inflammation drives unrelenting itch and sleep disturbance, which in turn impair daily functioning, mental health, and overall well-being.1,2 Many patients experience social withdrawal, reduced productivity, and heightened psychological stress when disease activity remains uncontrolled.1,2 As Prof. Kircik noted, AD was historically considered an “outside vs. inside” disease, reflecting the belief that barrier dysfunction and immune dysregulation were competing explanations.
It is now understood that the pathogenesis of AD reflects a dynamic interplay between skin barrier fragility and immune dysregulation.4 On one hand, genetic predispositions such as filaggrin mutations, along with reduced ceramide content and impaired lipid organization, weaken the epidermal barrier, allowing allergens to penetrate, microbes to colonize, and chronic itch to develop.3-5 Environmental triggers further compound this vulnerability.3 On the other hand, disturbances in the skin microbiome—mainly colonization by Staphylococcus aureus and Corynebacterium spp.—activate immune pathways that sustain inflammation.3 These two processes reinforce each other through type 2 inflammation.3
Th2 cytokines, particularly IL-4 and IL-13, suppress barrier protein expression, impair ceramide and lipid synthesis, and reduce antimicrobial peptide production, thereby facilitating microbial persistence and recurrent flares.3,6 Within the type 2 cytokine network, IL-13 has emerged as a pivotal upstream mediator.7 Beyond orchestrating immune responses, it directly affects keratinocyte function and ceramide levels, alters skin microbiome, and lipid metabolism, making it a key driver of both barrier dysfunction and chronic inflammation.7
Patients with AD require long-term management options beyond steroid treatments
Therapy for AD has historically relied on topical corticosteroids (TCS).1,6 When used appropriately—with the correct potency, duration, and site selection—they remain guideline-recommended and effective for acute flare control, particularly when paired with emollient therapy.6,8 However, their limitations are well recognized. While TCS suppress type 2 inflammation and provide some degree of symptomatic relief, short-term use has been shown to compromise the skin barrier by inhibiting epidermal lipid synthesis and other structural components.5,6 More fundamentally, corticosteroids suppress inflammation symptomatically but do not address the underlying cytokine-driven barrier dysfunction.6 This reliance on steroids, therefore, fails to fully break the pathogenic cycle.
Beyond these structural concerns, regulatory bodies such as Health Canada and the UK Medicines and Healthcare products Regulatory Agency (MHRA) have issued warnings regarding topical steroid withdrawal reactions, highlighting the need for safer, more durable treatment strategies.9,10 This gap between symptomatic control and durable disease modification has spurred the search for steroid-sparing strategies.7 Meeting the burden of AD means more than simply managing flares, explained Prof. Kircik, “patients need long-term control, reduced itch, and improved quality of life—milestones that steroids alone do not consistently achieve.”
The quest for durable, comprehensive disease control therapy
Dupilumab, the first biologic approved for AD, marked a major shift in the treatment landscape.7 By targeting IL-4Rα and thereby blocking both IL-4 and IL-13 signaling, it interrupts the type 2 inflammatory cascade, improves skin barrier function, and reduces symptoms.6,11 Prof. Kircik noted that while data from its clinical trials demonstrated notable efficacy compared to conventional therapies, there remain unmet needs that require attention.
Injection-site reactions are expected, but conjunctivitis has emerged as a notable and frequent adverse effect, observed in 3%-5% of patients.12 While severe AD itself is associated with ocular disease, conjunctivitis appears consistently higher with dupilumab.12,13 According to Prof. Kircik, the exact mechanism remains unclear but may relate to the underlying modulation of cytokine pathways. Other reported reactions include paradoxical exacerbation of AD, and more recently, musculoskeletal events such as arthralgia and psoriatic arthritis, which led to updates in the product label.12,14 Taken together, while dupilumab marked a breakthrough in AD care, treatment-limiting adverse events and incomplete responses highlight the continued need for therapies that provide more durable and comprehensive disease control.7,15 Prof. Kircik remarked that dupilumab is no longer the only option, and newer biologics with a different mechanism of action may offer a more beneficial efficacy and safety profile.
Lebrikizumab delivers deep and durable efficacy
Building on the limitations seen with existing therapies, lebrikizumab, a high-affinity monoclonal antibody that selectively targets IL-13 signaling while sparing the IL-13 decoy receptor, has emerged as a promising next-generation option.1,16 By focusing specifically on IL-13, it aims to interrupt a central driver of type 2 inflammation and restore barrier function more effectively.7
The pivotal ADvocate 1 and 2 trials enrolled patients with moderate-to-severe AD ([Investigator’s Global Assessment] IGA ≥3, Eczema Area and Severity Index [EASI] ≥16, ≥10% Body Surface Area [BSA]) who were naïve to other biologics.16
Both trials demonstrated rapid and clinically meaningful improvements by week 16:16
- IGA 0/1: 43.1% of lebrikizumab patients vs. 12.7% on placebo in ADvocate-1; 33.2% vs. 10.8% in ADvocate-2
- EASI-75: 58.8% vs. 16.2% in ADvocate-1; 52.1% vs. 18.1% in ADvocate-2
Reported adverse events during the induction period were mostly mild to moderate: conjunctivitis (~7%), nasopharyngitis (~4%), and headache (~4%) being the most common.16 Importantly, these gains were not transient but sustained through week 52 of the maintenance phase.17 Notably, patients who were withdrawn from lebrikizumab after achieving the per-protocol response at week 16 continued to maintain stable efficacy for up to Week 52.17 This durability of response, even in the absence of ongoing therapy, raises the possibility that lebrikizumab may exert a disease-modifying effect.17 With efficacy rates surpassing earlier benchmarks, lebrikizumab is helping to redefine treatment goals. As Prof. Kircik highlighted, “our bar should no longer be ‘almost clear’, deep response is what patients deserve.”
Stable disease control sustained over three years with lebrikizumab
The pivotal ADvocate trials demonstrated lebrikizumab’s ability to deliver rapid and deep responses by week 16, but the key question that followed was whether those gains could be maintained over time. This was the purpose of the ADjoin extension study, which enrolled responders from ADvocate 1 and 2 who had achieved either IGA 0/1 (response of clear or almost clear) or EASI-75 (75% improvement from baseline) at week 16 and assessed the long-term efficacy and safety of 3 years of continuous treatment with lebrikizumab.19 These patients transitioned into long-term maintenance, receiving lebrikizumab at either 250mg every two weeks (Q2W) or every four weeks (Q4W).19
By design, ADjoin provided a stringent test of durability: all participants entered with confirmed disease control, and outcomes were assessed over an extended follow-up period, including IGA and EASI responses, patient-reported measures of disease severity and quality of life, safety assessment, and the need for rescue therapy.19,20 Beyond sustaining disease control, lebrikizumab enabled truly deep responses.20 Through year 3, at least half of patients maintained complete skin clearance with IGA 0 (figure 1) and EASI-100 (figure 2), representing outcomes that exceed the conventional benchmarks of IGA 0/1 or EASI-90 reported in earlier biologic trials.12,20
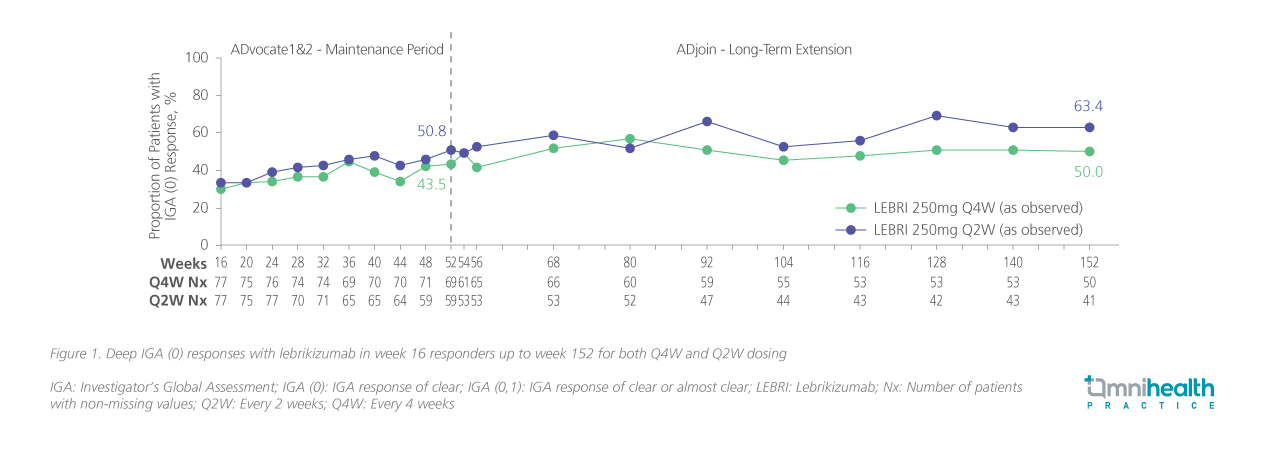
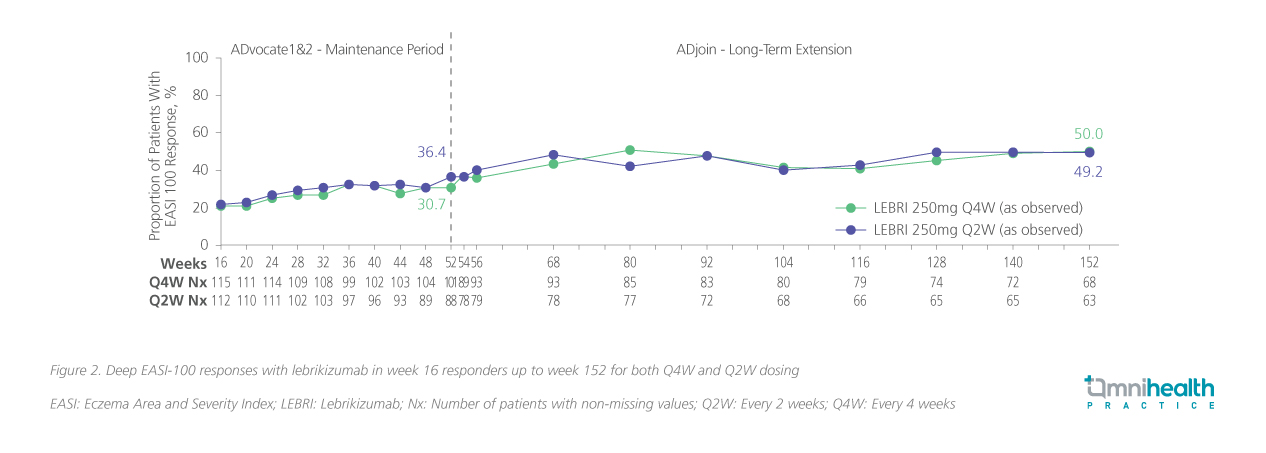
Quality-of-life measures mirrored these clinical outcomes. Patient-Oriented Eczema Measure (POEM) scores approached 0-1 for many patients, reflecting not only skin clearance but also tangible relief in daily living.20 Equally important, the majority of patients (>80%) remained free from TCS or other rescue therapies up to 152 weeks, highlighting lebrikizumab’s ability to deliver long-term disease control without adjunctive burden.19 Safety data reinforced this profile: no new safety signals emerged over time, adverse events were largely mild to moderate, and the incidence of serious adverse events remained low.19
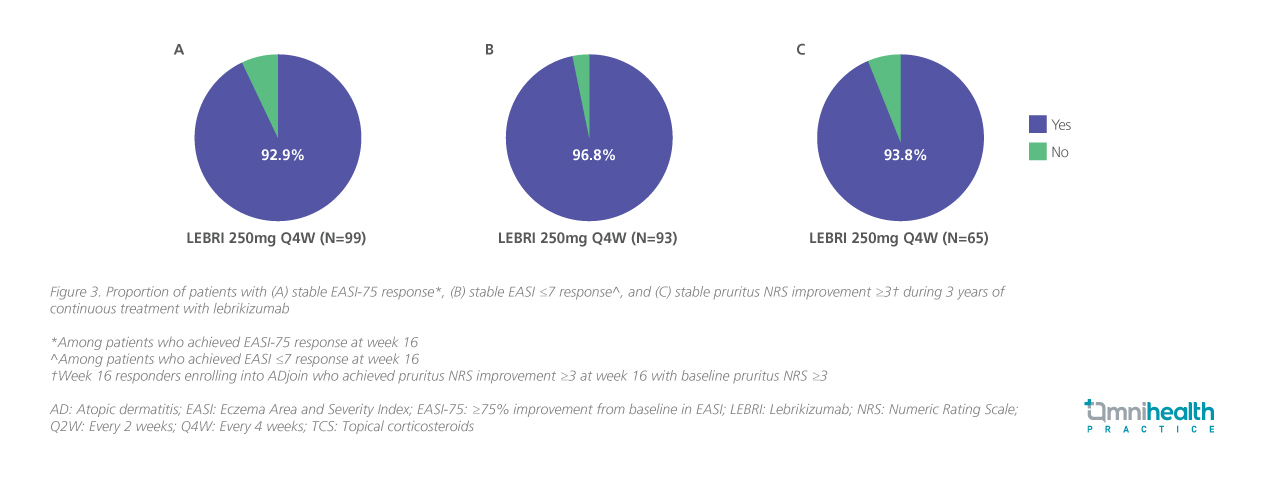
Beyond the depth of clearance, the long-term extension trial also highlighted the durability of lebrikizumab’s effects. With Q4W maintenance dosing, 92.9% of week 16 EASI-75 responders sustained their response through 3 years (figure 3A), while 96.8% of those with an EASI ≤7 at week 16 remained stable over the same period (figure 3B). In addition, 93.8% of patients who achieved a ≥3-point reduction in pruritus numeric rating scale (NRS) at week 16 maintained this improvement for 2 years (figure 3C).21 Taken together, these findings demonstrate that responses with lebrikizumab are not only deep, but remained stable over extended treatment. As Prof. Kircik emphasized, this represents a shift from managing flares to maintaining durable remission-like control.
Conclusion
The landscape of AD management is evolving rapidly. From an era when TCS were the primary option, treatment has advanced to biologics and small molecules that redefine achievable outcomes.1,3,6,7 Lebrikizumab’s trials (ADvocate 1 and 2, with long-term follow-up in ADjoin) provide rigorous, multi-year evidence of deep, durable, and stable responses.20,21 Reflecting on recent advances, Prof. Kircik highlighted that for clinicians, this translates into the ability to set higher treatment goals; for patients, it offers the possibility of complete remission, reduced disease burden with fewer flares, and less reliance on rescue interventions. With long-term data now extending to three years, lebrikizumab is emerging as more than biologic—raising the bar for AD management.20

