CONFERENCE UPDATE: ASCO 2025
Encorafenib + cetuximab + chemotherapy improves PFS and OS as first-line therapy in BRAF V600E-mutant mCRC: Results from the phase 3 BREAKWATER trial
STUDY DESIGN
BRAF V600E mutation occurs in approximately 8%-12% of patients with metastatic colorectal cancer (mCRC) and is associated with poor prognosis and limited response to standard chemotherapy-based regimens.1 Encorafenib is a highly selective, ATP-competitive BRAF inhibitor with prolonged pharmacodynamic activity.1 It has been approved for second- or later-line treatment of BRAF V600E-mutant mCRC.1 However, first-line treatment options specific to this aggressive molecular subtype remain limited.1
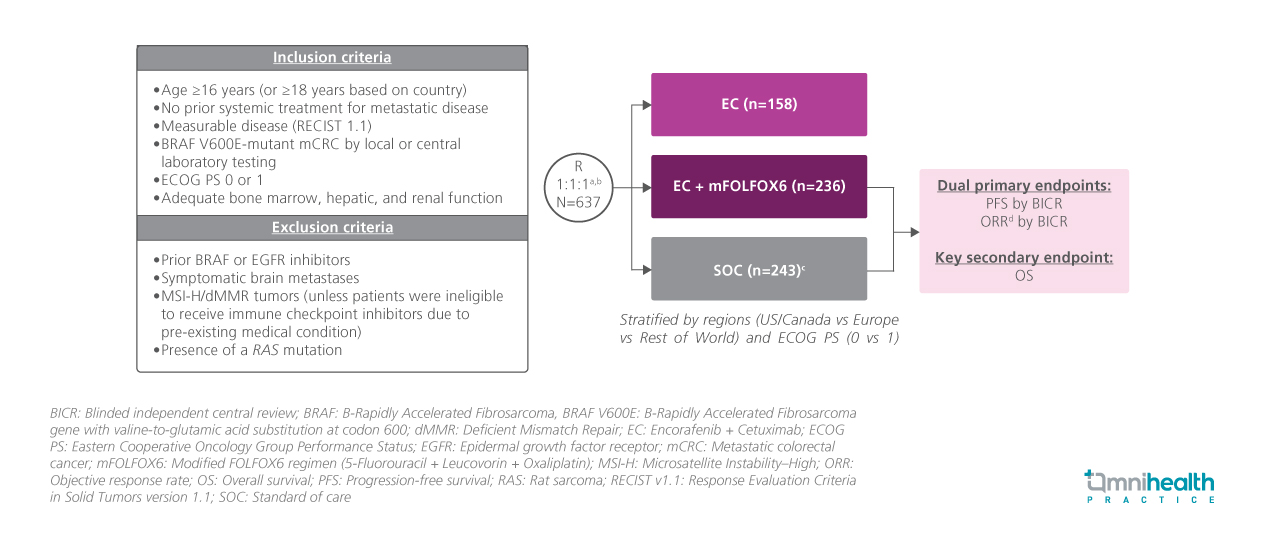
BREAKWATER is an ongoing, global, phase 3, open-label, randomized trial evaluating the efficacy and safety of encorafenib + cetuximab + chemotherapy as a first-line treatment for previously untreated patients with BRAF V600E-mutant mCRC.1 Eligible participants were adults aged ≥16 years with histologically confirmed metastatic colorectal adenocarcinoma harboring a BRAF V600E mutation, as determined by either local or central laboratory testing.1 All patients were systemically treatment-naïve in the metastatic setting and required measurable disease per RECIST v1.1.1 Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate bone marrow, hepatic, and renal function to support protocol-defined treatment.1
A total of 637 patients were enrolled and initially randomized into three treatment arms: encorafenib + cetuximab + mFOLFOX6 (E+C+chemo), encorafenib + cetuximab (E+C) or standard chemotherapy ± bevacizumab (SOC).1 Following a protocol amendment, the E+C arm was closed.1 Subsequently, patients were randomized 1:1 to receive either E+C+chemo (n=236) or SOC treatment (n=243).1 Baseline demographics and disease characteristics were generally well-balanced across treatment arms.1
The dual primary endpoints were progression-free survival (PFS) and objective response rate (ORR).1 Overall survival (OS) was a key secondary endpoint.1
FINDINGS
|
Primary endpoints: |
|
|
Secondary endpoint: |
|
|
Safety: |
|

