CONFERENCE UPDATE: ESGO 2024
HRQoL improvements with pembrolizumab + CCRT and EBRT in high-risk LACC patients in ENGOT-CX11/GOG-3047/KEYNOTE A18
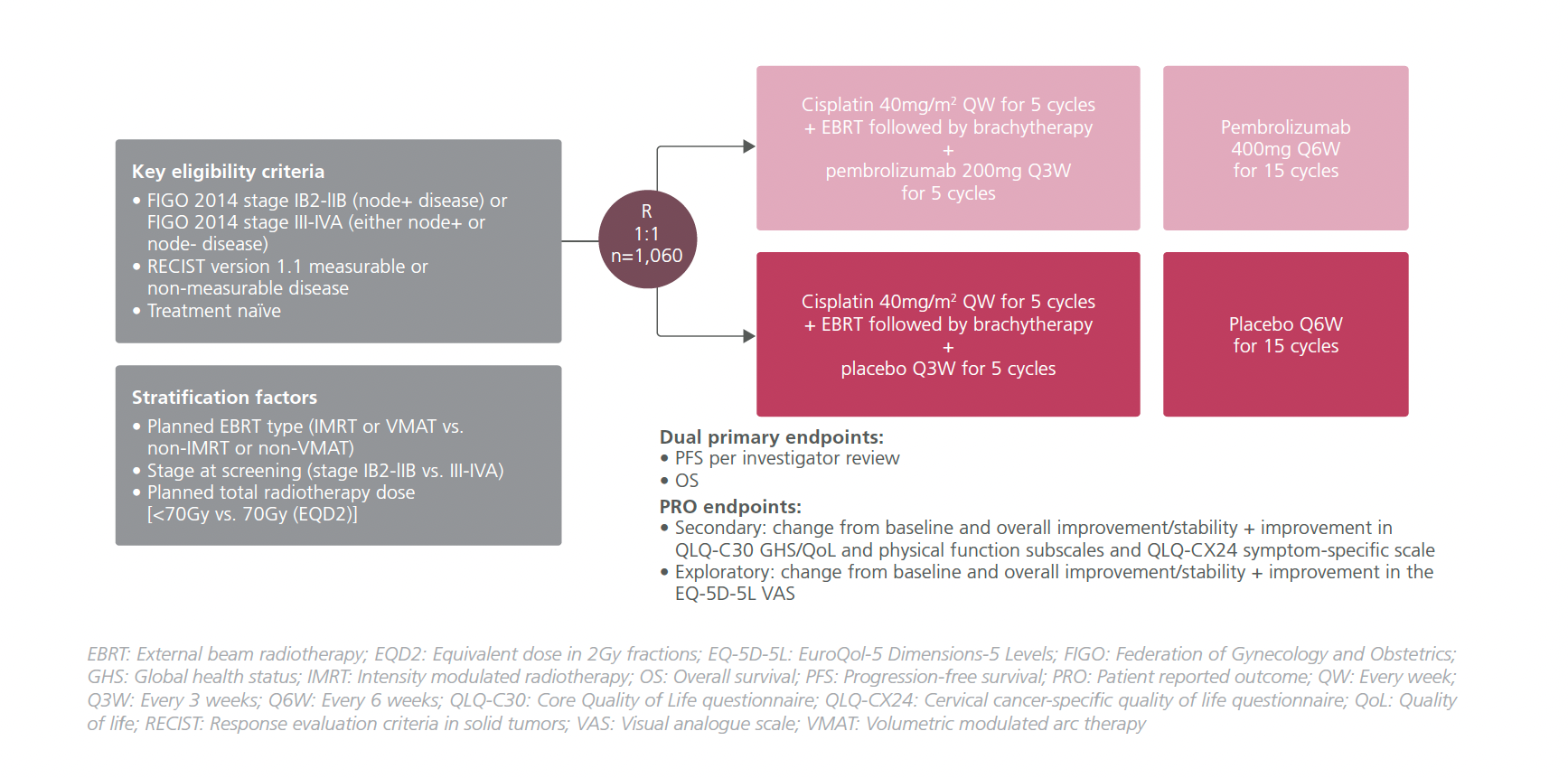
STUDY DESIGN
For over 2 decades, locally advanced cervical cancer (LACC) has been treated with external beam radiotherapy (EBRT) and concurrent chemotherapy, followed by brachytherapy.1 However, the toxicity associated with these treatments along with the burden of the disease itself have led to a decreased health-related quality of life (HRQoL) for patients.1 Studies conducted in both the clinical and preclinical settings have suggested the enhancing effect of immunotherapy on chemoradiotherapy (CCRT).1 Specifically, pembrolizumab, a programmed cell death protein 1 (PD-1) inhibitor has previously shown its efficacy and manageable safety profile in patients with cervical cancer.1 In the phase 3 ENGOT-cx11/GOG-3047/KEYNOTE-A18 study, the efficacy, safety and HRQoL patient-reported outcomes (PROs) of pembrolizumab and concurrent CCRT in patients with high-risk LACC had been reported.1
In the study, treatment-naïve LACC patients (n=1,060) with Federation of Gynecology and Obstetrics (FIGO) 2014 stages IB2-IIB node+ disease, or stage III-IVA disease regardless of measurable disease were included.1 They were randomized 1:1 to receive pembrolizumab 200mg Q3W or a matching placebo in addition to cisplatin 40mg/m2 weekly for a total of 5 cycles + EBRT followed by brachytherapy.1 Pembrolizumab 400mg Q6W or a matching placebo was continued for 15 cycles after that.1 The dual primary endpoints of the study were progression-free survival (PFS) per investigator review and overall survival (OS).1
In this presentation, the HRQoL PRO endpoints in terms of change from baseline and overall improvement or stability were reported at week 36.1 PROs were assessed at every treatment cycle before any treatment at follow-up until disease progression.1 However, no formal hypothesis testing was conducted for PRO analysis.1 They were assessed using prespecified questionnaires including the European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life questionnaire (QLQ-C30), EORTC cervical cancer-specific quality of life questionnaire (QLQ-CX24), and the EuroQol-5 Dimensions-5 Levels (EQ-5D-5L) visual analogue scale (VAS).1
FINDINGS
| PRO endpoints: |
|
|
|
| Other endpoints: |
|
|
|
“These data support pembrolizumab + CCRT as a new SoC for patients
with newly diagnosed,previously untreated, high-risk LACC”

