CONFERENCE UPDATE: ASH 2023
KRd offers durable survival benefits among patients with transplant-ineligible multiple myeloma
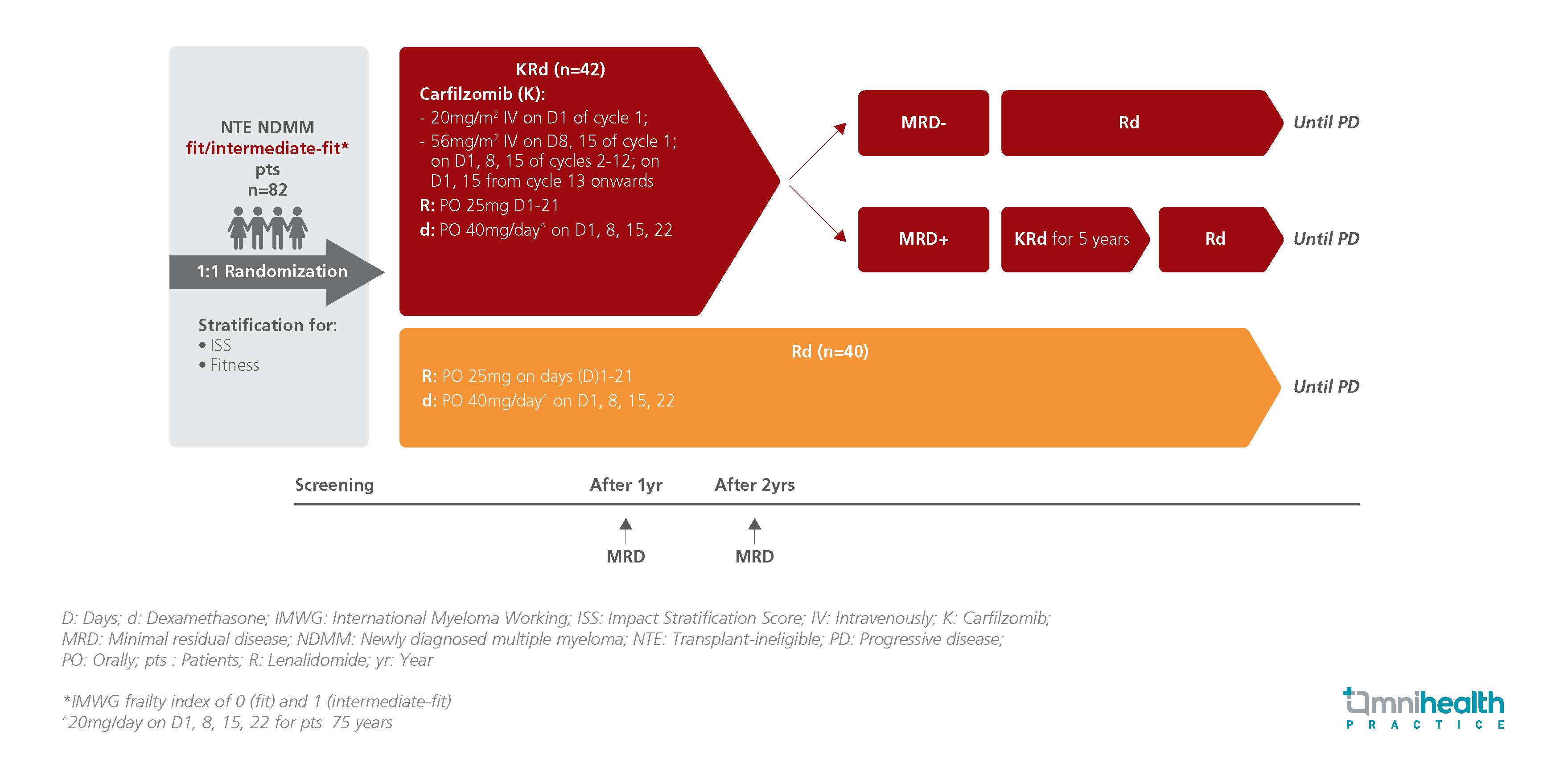
STUDY DESIGN
Lenalidomide-dexamethasone (Rd) had been the standard of care for transplant-ineligible (NTE) patients with newly diagnosed multiple myeloma (NDMM).1 In the FIRST trial, Rd showed a median progression-free survival (PFS) of 26 months, with a median PFS of 8.4 months in the high-risk subgroup.1 As the efficacy with Rd was still limited, there remains an unmet need for treatment options that offer longer clinical efficacy, particularly for fit to intermediate-fit patients, for whom the treatment goal is to achieve deep and prolonged response.1
The EMN20 trial was a multicenter, randomized, phase 3 trial that evaluated the clinical efficacy of the 3-drug regimen of carfilzomib plus lenalidomide-dexamethasone (KRd) compared to the Rd regimen in treating NTE NFMM fit to intermediate-fit patients.1 A total of 82 patients were enrolled and randomized to receive either KRd (n=42) or Rd (n=40) until disease progression.1 After 2 years of treatment, patients from the KRd arm who achieved minimal residual disease (MRD) negativity were switched to Rd alone while those that showed MRD positivity continued KRd for 5 years.1 The protocol was halted in Nov 2021, following the introduction of frontline daratumumab (Dara)-Rd.1
The primary endpoints were MRD status after 2 years of treatment, which was evaluated from MRD assessments performed in patients who achieved very good partial response (VGPR) during the first 2 years of the study period, and PFS.1 Key secondary endpoints included response rates (partial response [PR], very good partial response [VGPR], complete response [CR], overall survival (OS), and safety.1
|
Primary endpoint: |
|
|
Secondary endpoints: |
|
|
Safety: |
|
“The upfront treatment of KRd-Rd regimen had led to unexpectedly high rates of MRD negativity that delayed the progression among patients with NTE NDMM, which manifested into optimal clinical responses and progression-free survival benefits, regardless of age or size of genetic risk.”
Dr. Benedetto Bruno
Division of Hematology,
Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino,
University of Torino,
Torino, Italy

