CONFERENCE UPDATE: ESC 2023
Dapagliflozin demonstrated long-term efficacy and safety among HF patients with deteriorating kidney functions: Post-hoc analysis of DAPA-HF and DELIVER trial
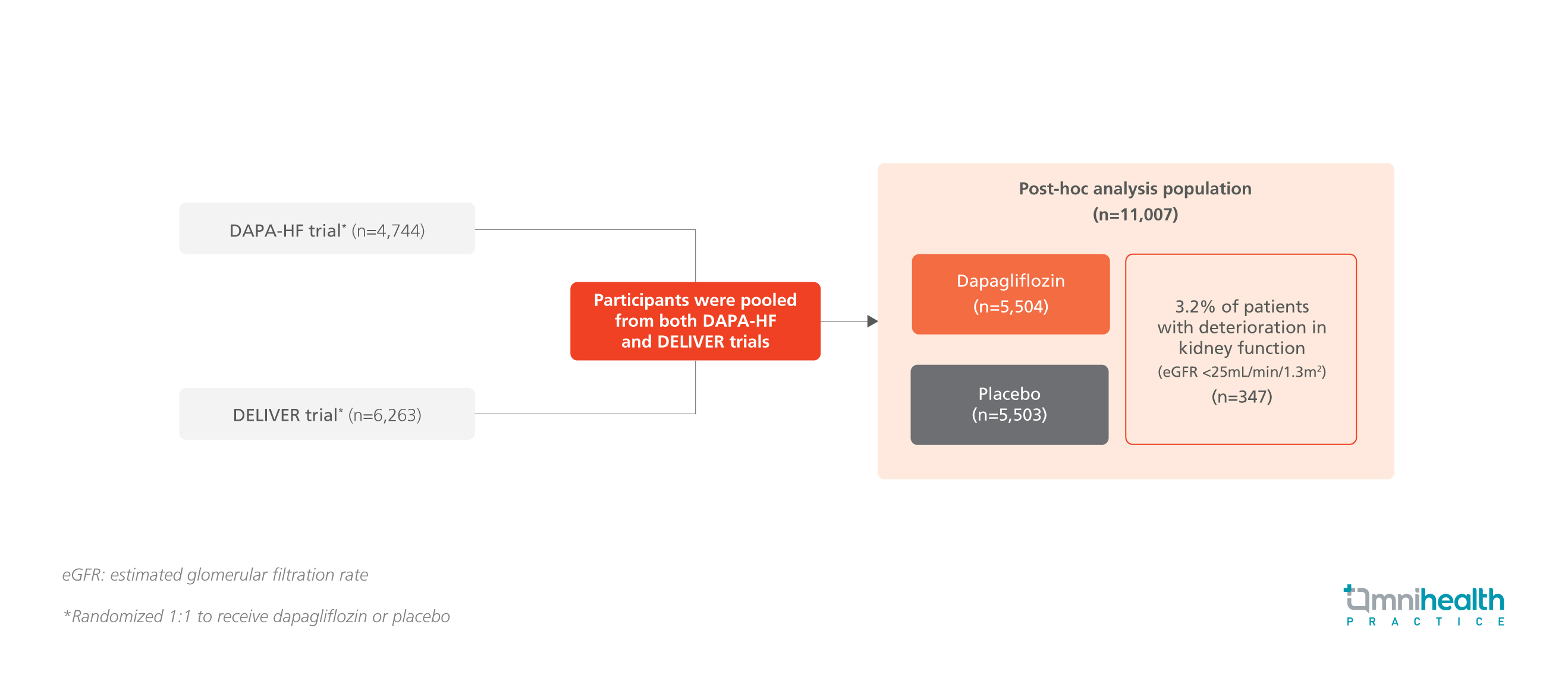
STUDY DESIGN
Dapagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, was proven to be safe and effective in chronic heart failure (HF) patients with an estimated glomerular filtration rate (eGFR) of 30mL/min/1.73m2 and 25mL/min/1.73m2 in the DAPA-HF and DELIVER trial respectively.1 A Post-hoc analysis was conducted among chronic HF patients in these 2 trials to determine the prognostic implications of deterioration of reduced kidney function (eGFR <25mL/min/1.73m2) and the clinical outcomes of dapagliflozin on this particular patient subgroup.1
This post-hoc analysis involved 11,007 randomized patients with chronic HF from the DAPA-HF and DELIVER trials, of which 50% of patients were categorized into the dapagliflozin cohort (n=5,504).1 Throughout the study period, 3.2% of patients experienced a deterioration of kidney function below eGFR <25mL/min/1.73m2 with a median time to deterioration of 121 days, while 74.4% and 73.5% of patients remained on their assigned study drug in the dapagliflozin and placebo cohorts respectively.1 The primary endpoints of this post-hoc analysis were the incidence of worsening HF events or cardiovascular (CV) death among the patient cohorts and the safety outcomes of dapagliflozin.1
FINDINGS
| Primary endpoint: |
|
|
|
| Safety: |
|
|
“Dapagliflozin was associated with more desirable outcomes regardless of deterioration of eGFR to 25mL/min/1.73m2. This benefit-to-risk ratio may favor continued treatment of dapagliflozin in patients with HF and progressively deteriorating kidney function”
Dr. Scott D. Solomon
Brigham and Women’s Hospital,
Boston, Massachusetts,
United States

