CONFERENCE UPDATE: ESC 2023
PROTECT-AR: Prospective, observational study in ACHD patients with atrial arrhythmia shows comparable efficacy and safety of apixaban for the prevention of thromboembolism vs. VKA
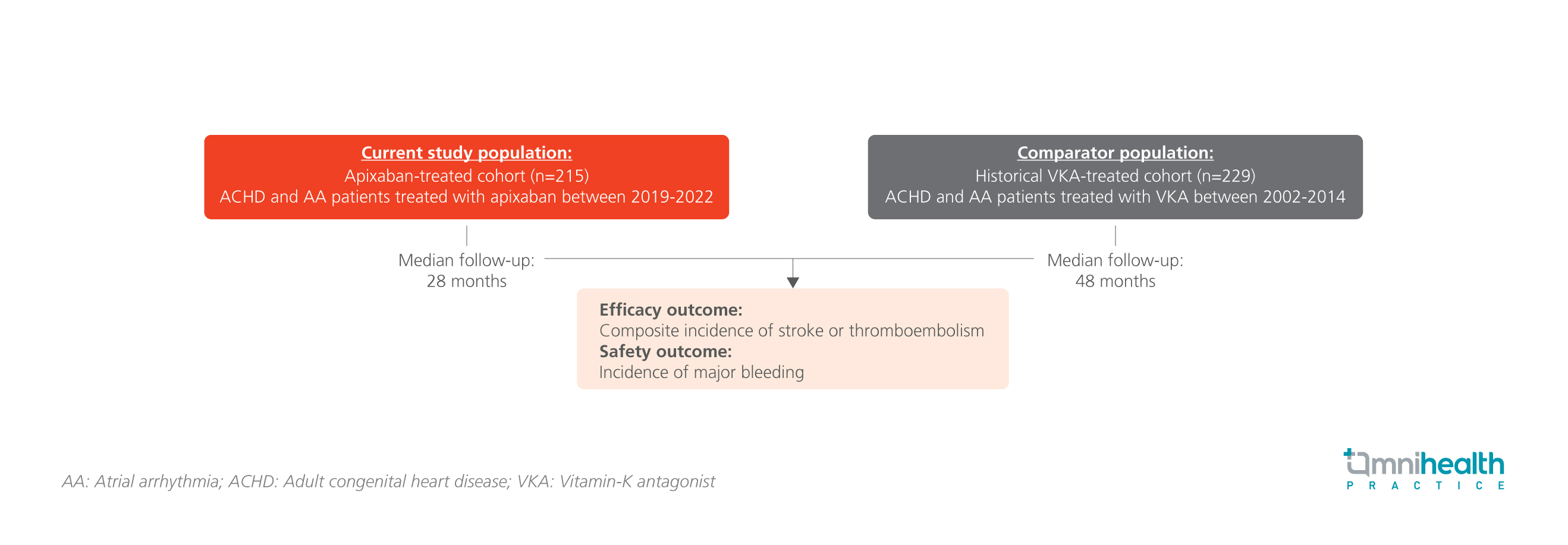
STUDY DESIGN
Patients with adult congenital heart disease (ACHD) and atrial arrhythmia (AA) are constantly burdened with an elevated risk of stroke and other thromboembolic events.1 Nevertheless, the current landscape of clinical data available on the use of non-vitamin K oral anticoagulants (NOACs) in this subgroup of patients remains imprecise and limited.1 To better address this knowledge gap and in turn better understand the use of NOACs in these patients, the PROTECT-AR study was designed to evaluate the efficacy and safety of apixaban, one of the NOACs, in the population of ACHD patients with comorbid AA.1
The clinical outcomes of 2 patient cohorts were evaluated and included and compared in this prospective, multicenter, observational study.1 The two cohorts were apixaban-treated cohort (n=215) and the historical vitamin-K antagonist (VKA)-treated cohort (n=229) respectively.1 Analysis was also conducted in a subgroup of patients on apixaban who had also recieved prior VKA treatment (n=73).1 The apixaban cohort received treatment from 2019 to 2022, with a median follow-up of 28 months.1 The historical VKA-treated cohort received treatment from 2002 to 2014, with a median follow-up of 48 months.1 It was noted that the patients from the apixaban cohort were significantly older than the historical VKA-cohort with a median age of 51 vs. 42 years old (p<0.001).1 They also had significantly higher incidences of diabetes mellitus, heart failure, and major or clinically relevant non-major bleeding events at baseline (p<0.001).1
The primary efficacy endpoint was the composite incidence of stroke or thromboembolism in the two cohorts, while the safety endpoint compared the incidence of major bleeding.1
FINDINGS
| Primary endpoint: |
|
|
|
| Safety: |
|
|
|
“In ACHD patients with AA treated with apixaban, the risk of major thromboembolic events and bleeding events are low and comparable to historical VKA clinical data”
Dr. Anastasios Kartas
AHEPA General Hospital of Aristotle University,
Thessaloniki, Greece

