CONFERENCE UPDATE: ESC 2023
Significant impact of sacubitril/valsartan on LV remodeling among HF patients with low blood pressure
Despite being prioritized as a first-line drug for heart failure (HF) patients with left ventricular ejection fraction (LVEF) <50%, the clinical efficacy of sacubitril/valsartan among HF patients with low blood pressure remains undetermined due to its reported potency in blood pressure reduction which precluded most clinical studies from continuing with sacubitril/valsartan.1 Hence, a retrospective study was conducted to evaluate the efficacy of sacubitril/valsartan on left ventricular (LV) remodeling for HF patients with LVEF <50% and systolic blood pressure ≤100mmHg.1
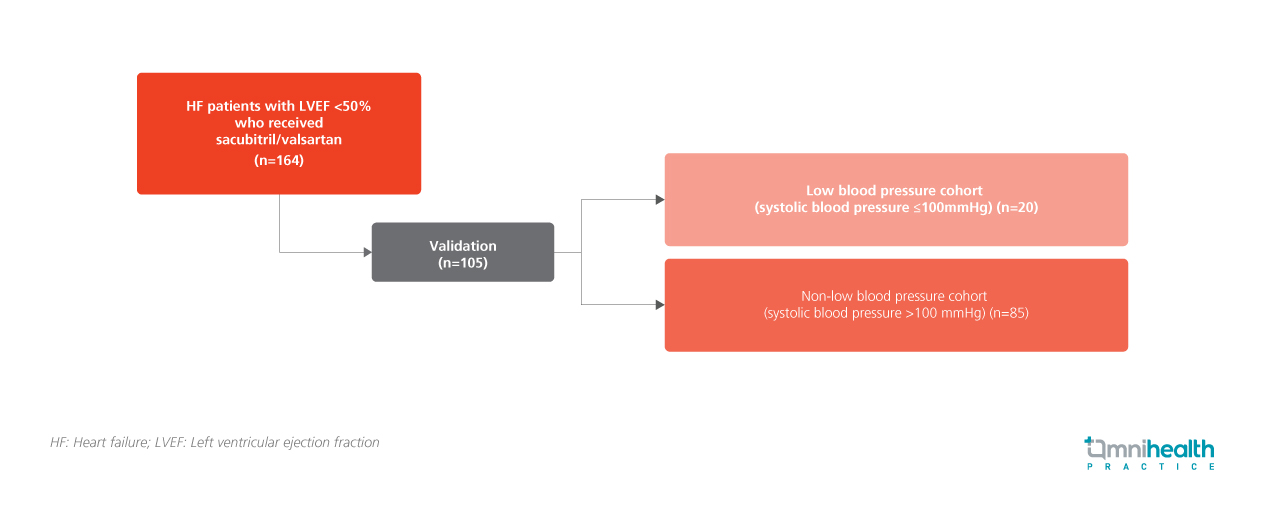
A total of 164 HF patients with LVEF <50% were recruited to receive sacubitril/valsartan between June 2020 and July 2022.1 Among the 105 validated patients, 20 with systolic blood pressure ≤100 mmHg were further categorized into the low blood pressure cohort, while the remainder (n=85) was considered as non-low blood pressure.1 Echocardiographic assessments were performed before treatment initiation as baseline and 9.5 ± 5.1 months after initiation of the maximum tolerated dose of sacubitril/valsartan as treatment outcome.1
|
Primary endpoints: |
|
|
Secondary endpoints: |
|
|
Safety |
|
“The findings of our study demonstrated that significant LV reverse remodeling outcomes were achieved in HF patients with LVEF <50% and systolic blood pressure ≤100mmHg after initiation of sacubitril /valsartan”
Dr. Yu Nishihara
Department of Internal Medicine,
Kobe University Graduate School of Medicine,
Kobe, Japan

