CONFERENCE UPDATE: ASCO 2023
Addition of olaparib and durvalumab to current SOC may improve PFS in patients with advanced OC without BRCA1/2 mutation
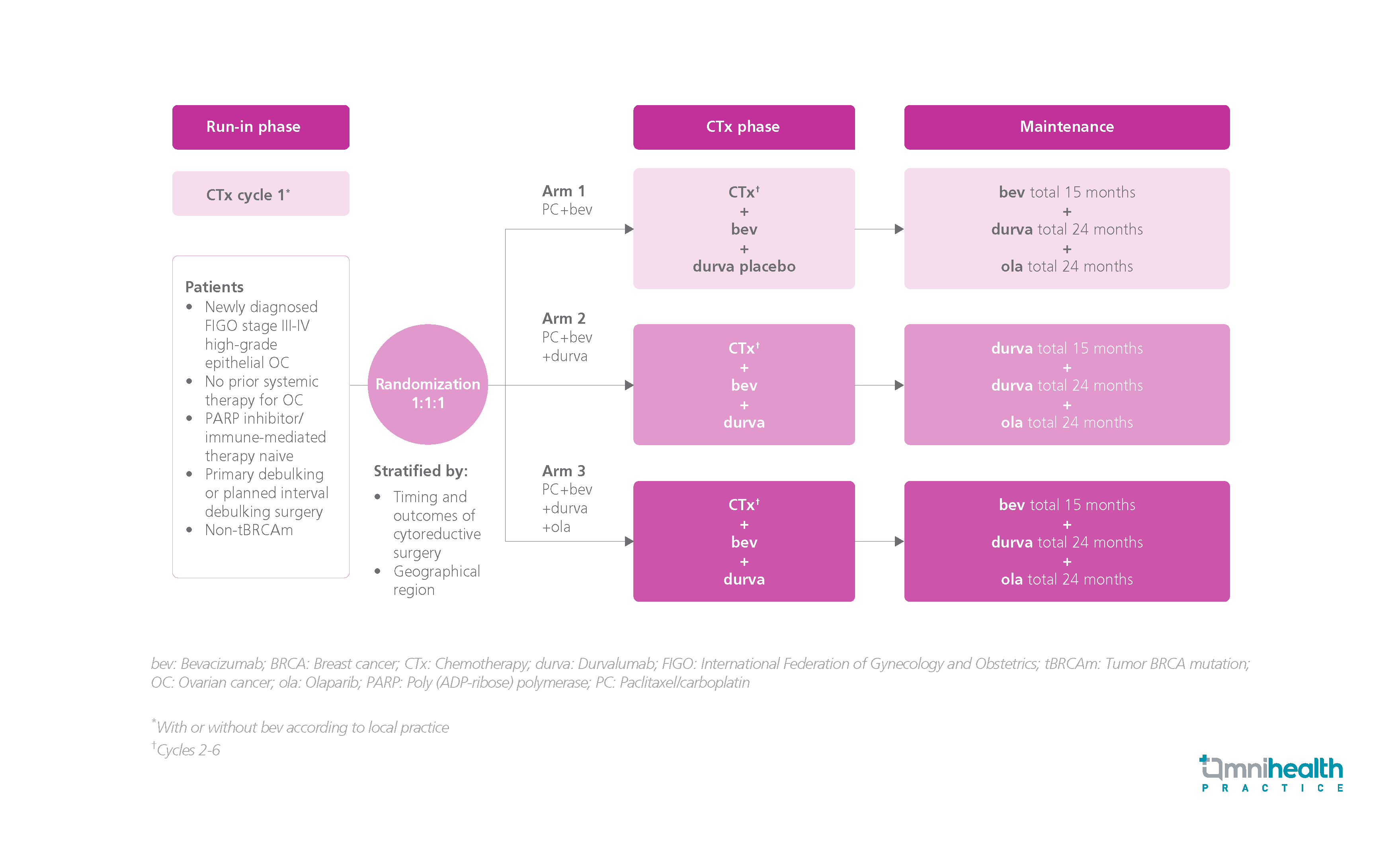
STUDY DESIGN
The combination of olaparib and bevacizumab as first-line maintenance therapy has demonstrated improved outcomes in advanced ovarian cancer (OC).1 Yet, the unmet needs remain among patients without breast cancer (BRCA) mutations.1 In the phase 2 MEDIOLA study, the clinical benefits of the combined therapy of bevacizumab, durvalumab, and olaparib among platinum-sensitive advanced OC patients without germline BRCA1/2 mutation were demonstrated, indicating the potential of applying immuno-oncology agents for improving the outcomes of these specific advanced OC patient subgroups.1
The DUO-O study is a randomized, phase 3 study designed to investigate the safety and efficacy of paclitaxel/carboplatin + bevacizumab + durvalumab followed by maintenance therapy with bevacizumab + durvalumab + olaparib in adult patients with newly diagnosed, International Federation of Gynecology and Obstetrics (FIGO) stage III to IV high-grade epithelial non-tumor BRCA mutation (tBRCAm) advanced OC.1 The patients did not receive prior systemic therapy for OC and were naïve to poly (ADP‐ribose) polymerase (PARP) inhibitors and immunotherapies.1 A total of 1,130 participants were recruited and randomized 1:1:1 into 3 arms: Arm 1 patients received chemotherapy + bevacizumab + durvalumab placebo, followed up with a 15-month maintenance therapy of bevacizumab + durvalumab placebo + olaparib placebo; arm 2 patients received chemotherapy + bevacizumab + durvalumab, followed up with a 15-month maintenance therapy of bevacizumab + a 24-month therapy of durvalumab + olaparib placebo; and arm 3 patients received chemotherapy + bevacizumab + durvalumab, followed up with a 15-month maintenance therapy of bevacizumab + 24-month therapies of durvalumab + olaparib.1 The treatment was continued until disease progression or treatment completion.1 The primary endpoint was progression-free survival (PFS), determined by Response Evaluation Criteria in Solid Tumors (RECIST) per investigator in arm 3 vs. arm 1.1 The positive preplanned PFS analysis was presented in the 2023 ASCO annual meeting.1 The DUO-O trial is still ongoing.1
FINDINGS
| Primary endpoint: |
|
|
|
|
| Secondary endpoints: |
|
|
| Safety: |
|
|
“We observe statistically significant and clinically meaningful improvements in PFS with first-line chemotherapy + bevacizumab + durvalumab, accompanied with a combined maintenance therapy of bevacizumab + durvalumab + olaparib”
Dr. Philipp Harter
Department of Gynecology & Gynecologic Oncology,
Evangelische Kliniken Essen-Mitte,
Essen, Germany

