MEETING HIGHLIGHT
More than glycated hemoglobin: Dapagliflozin preserves cardiorenal functions in patients with type 2 diabetes mellitus
In a recent webinar organized by the Hong Kong Society of Endocrinology, Metabolism and Reproduction Limited, Professor Jonathan Shaw, Deputy Director, Clinical and Population Health, at the Melbourne’s Baker Heart and Diabetes Institute, shared a new paradigm of diabetic care in which glycated hemoglobin (HbA1c) is no longer the only consideration for selecting therapeutic agents.1 With increasing evidence from multiple clinical trials, the treatment of type 2 diabetes mellitus (T2DM) should consider the cardiorenal risks independent of HbA1c level.1 With the DAPA-CKD study demonstrating the cardiorenal benefit of dapagliflozin, a sodium-glucose co-transporter inhibitors (SGLT2i), Prof. Shaw recommended all clinicians to actively consider SGLT2i for the treatment and prevention cardiorenal outcomes in patients with or without T2DM.2
Diabetic pathogenesis is associated with cardiomyopathy and nephropathy
Although it is conventionally believed that heart failure (HF) is a result of myocardial infarction (MI) or valvular disease, Prof. Shaw explained that diabetes can lead to HF through ischemia, coronary artery diseases and direct metabolic effects on myocardial function that can precede MI or angina.3 Notably, patients with diabetes have a greater risk of sudden cardiac death than those without diabetes (RR=1.75; 95% CI: 1.51-2.03; pheterogeneity=0.10) regardless of coexisting coronary heart diseases (RR=1.63; 95% CI: 1.36- 1.97) or HF (RR=1.85; 95% CI: 1.48-2.33).4 Interestingly, younger patients have a consistently greater excess risk of mortality, acute MI, stroke and HF than older patients irrespective of the number of underlying risk factors.5
On the other hand, diabetes is a key contributor to the global burden of end-stage renal disease (ESRD) with 50% of new ESRDs in Hong Kong in 2015 reporting diabetes as the primary cause.6 Prof. Shaw pointed out that the Australian data showed that although the rate of ESRD was stable among patients with type 1 diabetes, there was an annual increase of 2.2% in ESRD incidence among T2DM patients.7 Notably, the increased incidence of ESRD among T2DM patients is heavily driven by younger patients <50-years-old and by older patients >80-years-old.7
While being two separate sets of complications associated with diabetes, Prof. Shaw stated that the dysfunctions in the cardiovascular and renal systems are inextricably linked, sharing many common risk factors in both the acute and chronic phases.8 Where the dysfunction in one system can induce dysfunction in the other, acute decompensated congestive HF leads to acute kidney injury and induces chronic kidney disease (CKD).9 Reciprocally, impaired glomerular filtration alters sodium-potassium balance and results in arrhythmias and cardiomyopathy.9 Given the increased cardiovascular and renal risks among younger patients, Prof. Shaw emphasized that T2DM should be treated early to achieve better disease control. “In Hong Kong, more and more younger adults are presenting with T2DM. [The risk of cardiorenal diseases] in 5 to 10 years will further increase unless intervention is introduced early,” reiterated Prof. Shaw.
Unmet needs with HbA1c as the treatment target for patients with T2DM
Where hyperglycemia as assessed by HbA1c is considered a statistically independent and potentially modifiable risk factor for cardiovascular disease, aggressive glucose-lowering treatment was showed to only reduce the risk of major cardiovascular events by 9% (HR=0.91; 95% CI: 0.84-0.99) and MI by 15% (HR=0.85; 95% CI: 0.76-0.94) when compared to less-intensive treatment.10 Contrarily, no benefit was observed in all-cause mortality, HF or stroke when more intensive glycemic control was used.10 To address the cardiorenal risks of T2DM, a meta-analysis of randomized, placebo-controlled, cardiovascular outcome trials showed that SGLT2i can reduce the risk of hospitalization due to HF or cardiovascular death (HR=0.76; 95% CI: 0.69-0.84; p<0.0001) regardless of the patients with atherosclerotic cardiovascular disease (ASCVD) or with multiple risk factors (HR=0.84; 95% CI: 0.69-1.01; pinteraction=0.41).11 Similarly, SGLT2i improved renal outcomes among T2DM patients that was consistent across patients with low estimated glomerular filtration rate (eGFR) of <60mL/min/m2 (HR=0.67; 95% CI: 0.51-0.89; p=0.0054) and high eGFR of ≥90mL/min/m2 (HR=0.44; 95% CI: 0.32-0.59; p<0.0001).11“While [the benefit] was even more impressive for patients that had a baseline eGFR of ≥90mL/min/m2, SGLT2i had a substantial benefit for all patients in terms of renal outcomes,” noted Prof. Shaw who continued to point out the inadequacy of using HbA1c as the sole target for managing the cardiorenal risks of T2DM patients.
Dapagliflozin prevents adverse renal outcomes equally among patients with or without diabetes
While SGLT2i had improved cardiorenal outcomes among T2DM patients, Prof. Shaw noted that the DECLARE-TIMI 58 study (n=17,160) had covered the largest number of participants with multiple risk factors for ASCVD, but without established symptomatic cardiovascular disease (59%).12 In this study, dapagliflozin significantly reduced the risk of cardiovascular death or hospitalization for HF among T2DM patients with or without ASCVD (HR=0.83; 95% CI: 0.73-0.95; p=0.005).12 Also, dapagliflozin significantly reduced the risk of renal composite outcome either with (HR=0.76; 95% CI: 0.67-0.87; p<0.0001) or without accounting for cardiovascular death (HR=0.53; 95% CI: 0.43-0.66; p<0.0001).12,13 While a numerical benefit was observed among patients with a higher baseline eGFR, the renal protective benefit of dapagliflozin was consistent among all patients with eGFR <60 or ≥90mL / min /m2 ( pinteraction=0.87).13 Importantly, dapagliflozin was associated with a 46% reduction in sustained eGFR decline by at least 40% to <60ml /min /1.73m2 (HR=0.54; 95% CI: 0.43-0.67; p<0.0001) and a significantly lower risk of ESRD or renal death (HR=0.41; 95% CI: 0.20- 0.82; p=0.012) when compared to placebo.13 “The change in eGFR was considerably slower in patients receiving dapagliflozin than those receiving placebo, indicating the benefit of dapagliflozin not only in patients with ESRD but also across [patients] with a spectrum of renal function,” highlighted Prof. Shaw when considering the prevention and treatment of cardiorenal outcomes with dapagliflozin.
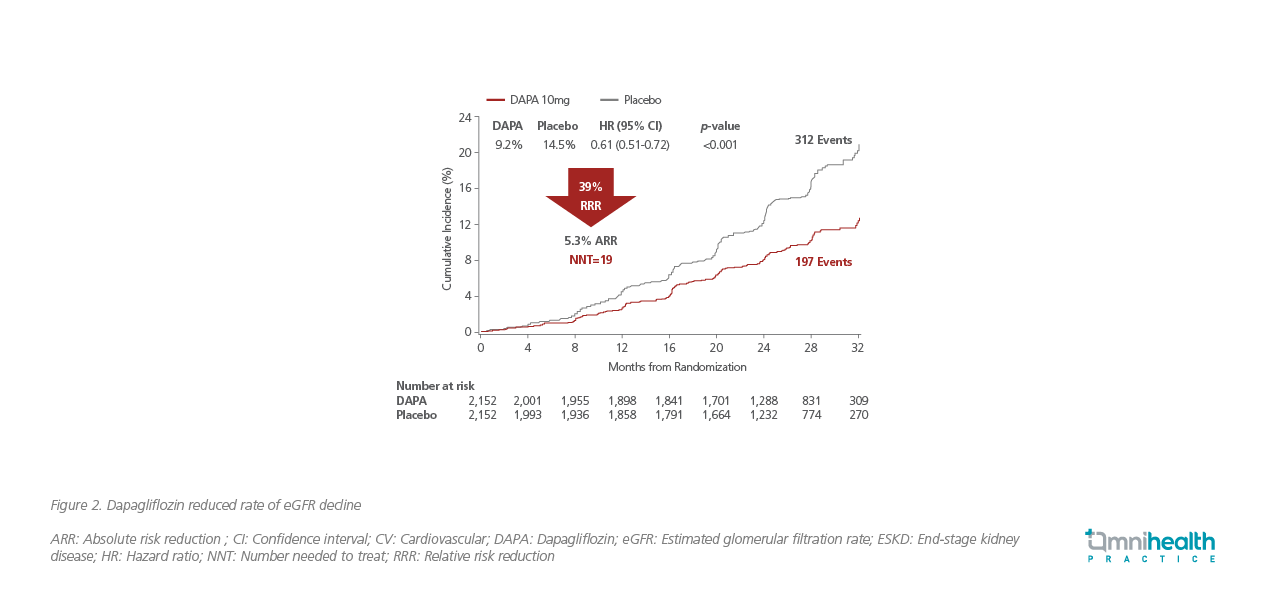
To further evaluate the renal-specific benefit of dapagliflozin in patients without T2DM, the randomized, double-blind, placebo-controlled, multicenter DAPA-CKD study (n=4,304) recruited patients with or without T2DM to assess the primary composite outcome of sustained ≥50% eGFR decline, ESRD, renal or cardiovascular death (Figure 1). 2

After a median follow-up of 2.4 years, dapagliflozin significantly slowed the progression of kidney disease with a 39% relative risk reduction in the primary composite outcome when compared to placebo (HR=0.61; 95% CI: 0.51-0.72; p=0.001; Figure 2).2 Moreover, dapagliflozin also reduced the composite of cardiovascular death or hospitalization for HF (HR=0.71; 95% CI: 0.55-0.92; p=0.009) and allcause mortality (HR=0.69; 95% CI: 0.53-0.88; p=0.004).2 Interestingly, Prof. Shaw remarked that only 67.6% of patients randomized to the dapagliflozin group had T2DM, indicating that the renal benefit of dapagliflozin was independent of its glucose-lowering effect and was consistent across patients with (HR=0.64; 95% CI: 0.52-0.79) or without T2DM (HR=0.50; 95% CI: 0.35-0.72).2 “Now, there is more evidence suggesting that hyperglycemia is not required to obtain a renal benefit from SGLT2i, supporting the need of change in the treatment paradigm of diabetes,” summarized Prof. Shaw.
In addition to renal-specific benefits, the DAPA-HF study (n=4,744) also demonstrated the cardioprotective benefit of dapagliflozin in reducing the primary outcome of worsening HF or cardiovascular diseases when compared to placebo (HR=0.74; 95% CI: 0.65-0.85; p<0.001).14 Similar to the DAPA-CKD study, Prof. Shaw noted that the benefit in primary outcome was virtually identical in patients with (HR=0.75; 95% CI: 0.63-0.90) or without diabetes (HR=0.73; 95% CI: 0.60-0.88), supporting the cardiorenal benefit of dapagliflozin being independent of glycemic control.14
In terms of safety, Prof. Shaw remarked that patients receiving sulfonylurea or insulin may experience hypoglycemia with the addition of SGLT2i. However, this adverse outcome should not be attributed directly to SGLT2i as multiple clinical trials had demonstrated a comparable safety profile between dapagliflozin and placebo.14,15 If hypoglycemia does occur in this setting, a reduction in the dose of sulfonylurea or insulin, rather than stopping the SGLT2i, is appropriate. Where volume depletion is a common adverse event experienced by elderly T2DM patients, the DECLARE-TIMI 58 study showed that the rates of amputation, fracture, volume depletion, and urinary tract infection were balanced between dapagliflozin and placebo.12 As a once daily oral tablet with proven efficacy and safety, Prof. Shaw considered SGLT2i including dapagliflozin to be a straightforward treatment that should be considered for all indicated patients to treat and prevent cardiorenal outcomes.
Other than dapagliflozin, glucagon-like peptide-1 receptors agonists (GLP1-RA) were also shown to reduce the renal composite outcome of sustained eGFR decline ≥40%, ESRD or all-cause death by 17% when compared to placebo (HR=0.83; 95% CI: 0.75-0.92; p<0.001).16 However, when evaluating specifically for the worsening of kidney function, the benefit of GLP1-RA based on eGFR decline was not statistically significant and was less pronounced than with SGLT2i.17 “While there is probably a modest benefit for renal function [with GLP1-RA], the benefit is not yet proven,” commented Prof. Shaw.
A new treatment paradigm for T2DM patients with high cardiorenal risks
For the past few decades, Prof. Shaw explained that the treatment paradigm for T2DM had focused on glycemic control with HbA1c as the trigger for treatment change. Under this premise, drug selection was solely based on the potency in lowering HbA1c with risks of MI and stroke being secondary considerations. However, as cardiorenal risks can manifest even when the HbA1c level is at target, Prof. Shaw emphasized that a change in the treatment paradigm would be needed to re-focus the trigger on the elevated risks of adverse clinical outcomes regardless of the adequacy in glycemic control (Figure 3).
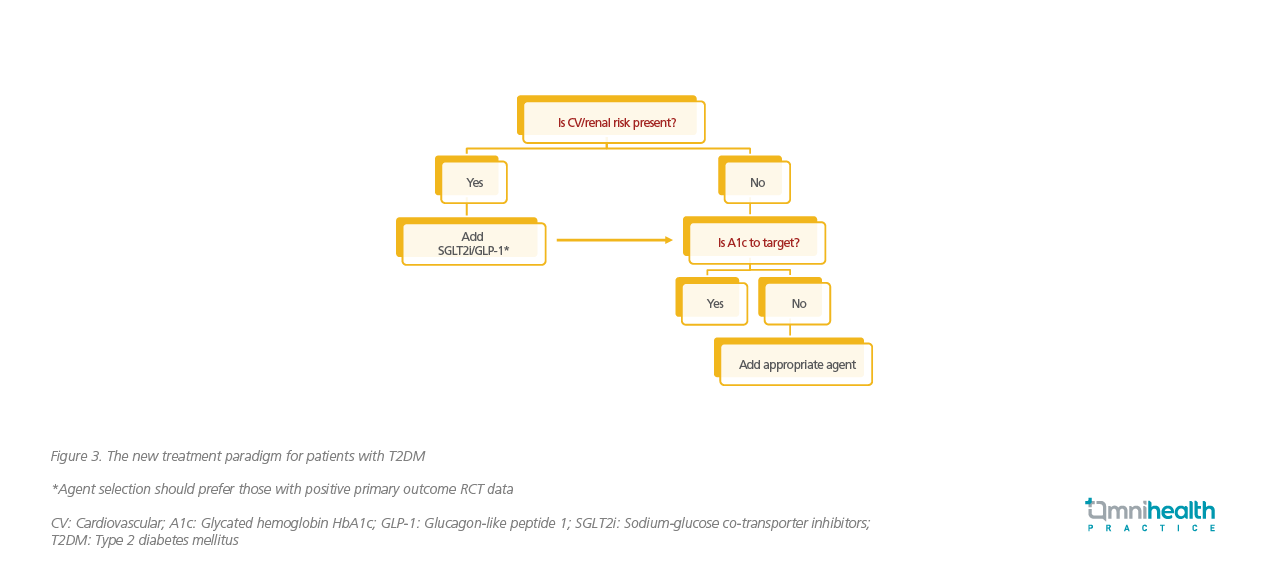
At first presentation, Prof. Shaw recommended all patients to be assessed on cardiorenal risks and given SGLT2i or GLP1-RA especially when prior cardiovascular disease, HF, reduced eGFR or albuminuria are identified. After addressing these cardiorenal risks, the patients’ HbA1c level should be assessed and an appropriate agent should be added to achieve the HbA1c target. When selecting treatment, Prof. Shaw noted that SGLT2i is preferred in patients who are primarily identified with an elevated risk of renal or HF outcomes. For those with underlying risks in MI or stroke, SGLT2i and GLP1-RA can be equally considered. Importantly, the treatment agents of choice should have positive primary outcomes as demonstrated in their respective randomized controlled trials. “While it is probable that the SGLT2i benefit is a class effect instead of a molecular effect … we should go for agents which have primary data to support their benefits in their own respective trials rather than selecting agents that do not,” restated Prof. Shaw.
With demonstrated significant cardiorenal benefits in the DECLARE-TIMI 58, DAPA-CKD and DAPA-HF studies, dapagliflozin is now one of the SGLT2is recommended by the American Diabetes Association (ADA) for the management of diabetic patients at risk of cardiorenal outcomes.1 According to the ADA Standards of Care 2021, all patients should be evaluated on the indicators of ASCVD, CKD or HF independently of HbA1c before considering treatment.1 For patients with increased risks of heart failure with reduced ejection fraction (HFrEF) or CKD, SGLT2i are preferred with proven evidence to reduce HFrEF and CKD progression.1 For patients with high-risks of ASCVD, SGLT2i and GLP1-RA can be equally considered.1 Under this new risk-based paradigm, Prof. Shaw elaborated that SGLT2i and GLP1-RA should be considered based on the risk factors present before considering HbA1c. In fact, all patients with T2DM and additional cardiovascular risks should actively consider SGLT2i or GLP1-RA, with SGLT2i being preferred in those with elevated risks of HF and CKD.
While local practitioners in Hong Kong typically prescribe SGLT2i to control bodyweight and reduce cardiovascular outcomes and/ or renal dysfunctions, Prof. Shaw discouraged a low carbohydrate diet in combination with SGLT2i when managing bodyweight. Importantly, Prof. Shaw recommended all clinicians to first assess the patient’s underlying cardiorenal risks and consider SGLT2i or GLP1-RA before addressing HbA1c or bodyweight-related issues. When selecting the most appropriate SGLT2i, Prof. Shaw commented that dapagliflozin is safe and efficacious in patients with a baseline eGFR as low as 30mL/min/1.73m2 or are at risk of cardiorenal morbidities regardless of being younger or older than 75-years-old.
Conclusion
To better manage the long-term disease outcome of T2DM patients, a paradigm shift from monitoring solely the HbA1c level to the presence of cardiorenal risks would be required. Based on ADA recommendations, SGLT2i should be considered for all patients at increased risks of HFrEF or CKD.1 With the strongest evidence available as demonstrated in the DECLARE-TIMI 58, DAPA-CKD and DAPA-HF studies, dapagliflozin is now indicated to treat symptomatic chronic heart failure with reduced ejection fraction regardless of the T2DM status.2

